Alvaxanthone, a Thymidylate Synthase Inhibitor with Nematocidal and Tumoricidal Activities
- PMID: 32586022
- PMCID: PMC7356228
- DOI: 10.3390/molecules25122894
Alvaxanthone, a Thymidylate Synthase Inhibitor with Nematocidal and Tumoricidal Activities
Abstract
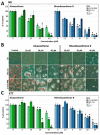
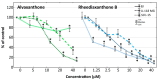
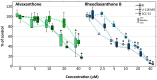
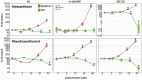
With the aim to identify novel inhibitors of parasitic nematode thymidylate synthase (TS), we screened in silico an in-house library of natural compounds, taking advantage of a model of nematode TS three-dimensional (3D) structure and choosing candidate compounds potentially capable of enzyme binding/inhibition. Selected compounds were tested as (i) inhibitors of the reaction catalyzed by TSs of different species, (ii) agents toxic to a nematode parasite model (C. elegans grown in vitro), (iii) inhibitors of normal human cell growth, and (iv) antitumor agents affecting human tumor cells grown in vitro. The results pointed to alvaxanthone as a relatively strong TS inhibitor that causes C. elegans population growth reduction with nematocidal potency similar to the anthelmintic drug mebendazole. Alvaxanthone also demonstrated an antiproliferative effect in tumor cells, associated with a selective toxicity against mitochondria observed in cancer cells compared to normal cells.
Keywords: cytotoxicity; inhibitor; nematocidal activity; thymidylate synthase; xanthones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Antitumor and anti-nematode activities of α-mangostin.Eur J Pharmacol. 2019 Nov 15;863:172678. doi: 10.1016/j.ejphar.2019.172678. Epub 2019 Sep 19. Eur J Pharmacol. 2019. PMID: 31542481
-
Design, synthesis and biological evaluation of novel uracil derivatives bearing 1, 2, 3-triazole moiety as thymidylate synthase (TS) inhibitors and as potential antitumor drugs.Eur J Med Chem. 2019 Jun 1;171:282-296. doi: 10.1016/j.ejmech.2019.03.047. Epub 2019 Mar 24. Eur J Med Chem. 2019. PMID: 30927565
-
Discovery of N-phenyl-(2,4-dihydroxypyrimidine-5-sulfonamido) phenylurea-based thymidylate synthase (TS) inhibitor as a novel multi-effects antitumor drugs with minimal toxicity.Cell Death Dis. 2019 Jul 11;10(7):532. doi: 10.1038/s41419-019-1773-0. Cell Death Dis. 2019. PMID: 31296849 Free PMC article.
-
Thymidylate synthase inhibitors.Clin Cancer Res. 1996 Feb;2(2):227-43. Clin Cancer Res. 1996. PMID: 9816165 Review.
-
Folate-based thymidylate synthase inhibitors as anticancer drugs.Ann Oncol. 1995 Nov;6(9):871-81. doi: 10.1093/oxfordjournals.annonc.a059353. Ann Oncol. 1995. PMID: 8624289 Review.
Cited by
-
Rosmarinus Officinalis L. essential oil: in Silico molecular docking and in vivo nematocidal activity on intestinal and muscular trichinellosis.BMC Complement Med Ther. 2025 Jul 9;25(1):250. doi: 10.1186/s12906-025-04996-7. BMC Complement Med Ther. 2025. PMID: 40634875 Free PMC article.
-
Recent Trends in Enzyme Inhibition and Activation in Drug Design.Molecules. 2020 Dec 22;26(1):17. doi: 10.3390/molecules26010017. Molecules. 2020. PMID: 33375159 Free PMC article.
-
Biotin Transport-Targeting Polysaccharide-Modified PAMAM G3 Dendrimer as System Delivering α-Mangostin into Cancer Cells and C. elegans Worms.Int J Mol Sci. 2021 Nov 29;22(23):12925. doi: 10.3390/ijms222312925. Int J Mol Sci. 2021. PMID: 34884739 Free PMC article.
References
-
- Rathod P.K. Antimalarial Agents Directed at Thymidylate Synthase. J. Pharm. Pharmacol. 1997;49:65–69. doi: 10.1111/j.2042-7158.1997.tb06163.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

