Capsaicin-Sensitive Sensory Nerves and the TRPV1 Ion Channel in Cardiac Physiology and Pathologies
- PMID: 32586044
- PMCID: PMC7352834
- DOI: 10.3390/ijms21124472
Capsaicin-Sensitive Sensory Nerves and the TRPV1 Ion Channel in Cardiac Physiology and Pathologies
Abstract
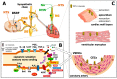
Cardiovascular diseases, including coronary artery disease, ischemic heart diseases such as acute myocardial infarction and postischemic heart failure, heart failure of other etiologies, and cardiac arrhythmias, belong to the leading causes of death. Activation of capsaicin-sensitive sensory nerves by the transient receptor potential vanilloid 1 (TRPV1) capsaicin receptor and other receptors, as well as neuropeptide mediators released from them upon stimulation, play important physiological regulatory roles. Capsaicin-sensitive sensory nerves also contribute to the development and progression of some cardiac diseases, as well as to mechanisms of endogenous stress adaptation leading to cardioprotection. In this review, we summarize the role of capsaicin-sensitive afferents and the TRPV1 ion channel in physiological and pathophysiological functions of the heart based mainly on experimental results and show their diagnostic or therapeutic potentials. Although the actions of several other channels or receptors expressed on cardiac sensory afferents and the effects of TRPV1 channel activation on different non-neural cell types in the heart are not precisely known, most data suggest that stimulation of the TRPV1-expressing sensory nerves or stimulation/overexpression of TRPV1 channels have beneficial effects in cardiac diseases.
Keywords: acute myocardial infarction; arrhythmia; atherosclerosis; capsaicin; cardioprotection; heart failure; ischemic heart disease; transient receptor potential vanilloid type 1 (TRPV1) receptor.
Conflict of interest statement
P.F. is a founder and CEO, and P.B. is employed by Pharmahungary, a group of R&D companies. Z.H. is the founder and strategic director of PharmInVivo Ltd. The funders had no role in the collection, or interpretation of data; in the writing of the manuscript, or in the decision to publish the manuscript. Otherwise, the authors state that there is no conflict of interest.
Figures
References
-
- Jancsó N. The Pharmacology of Pain. Pergamon Press; Oxford, UK: 1968. Desensitization with Capsaicin as a Tool for Studying the Function of Pain Receptors; pp. 33–55.
-
- Jancsó G., Hökfelt T., Lundberg J.M., Kiraly E., Halász N., Nilsson G., Terenius L., Rehfeld J., Steinbusch H., Verhofstad A., et al. Immunohistochemical Studies on the Effect of Capsaicin on Spinal and Medullary Peptide and Monoamine Neurons Using Antisera to Substance P, gastrin/CCK, Somatostatin, VIP, Enkephalin, Neurotensin and 5-hydroxytryptamine. J. Neurocytol. 1981;10:963–980. doi: 10.1007/BF01258524. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

