MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes
- PMID: 32586047
- PMCID: PMC7352860
- DOI: 10.3390/ijms21124471
MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes
Abstract
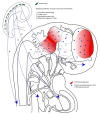
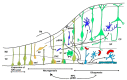
The signaling pathway of the microtubule-associated protein kinase or extracellular regulated kinase (MAPK/ERK) is a common mechanism of extracellular information transduction from extracellular stimuli to the intracellular space. The transduction of information leads to changes in the ongoing metabolic pathways and the modification of gene expression patterns. In the central nervous system, ERK is expressed ubiquitously, both temporally and spatially. As for the temporal ubiquity, this signaling system participates in three key moments: (i) Embryonic development; (ii) the early postnatal period; and iii) adulthood. During embryonic development, the system is partly responsible for the patterning of segmentation in the encephalic vesicle through the FGF8-ERK pathway. In addition, during this period, ERK directs neurogenesis migration and the final fate of neural progenitors. During the early postnatal period, ERK participates in the maturation process of dendritic trees and synaptogenesis. During adulthood, ERK participates in social and emotional behavior and memory processes, including long-term potentiation. Alterations in mechanisms related to ERK are associated with different pathological outcomes. Genetic alterations in any component of the ERK pathway result in pathologies associated with neural crest derivatives and mental dysfunctions associated with autism spectrum disorders. The MAP-ERK pathway is a key element of the neuroinflammatory pathway triggered by glial cells during the development of neurodegenerative diseases, such as Parkinson's and Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis, as well as prionic diseases. The process triggered by MAPK/ERK activation depends on the stage of development (mature or senescence), the type of cellular element in which the pathway is activated, and the anatomic neural structure. However, extensive gaps exist with regards to the targets of the phosphorylated ERK in many of these processes.
Keywords: hippocampus; learning; long term depression; long term potentiation; memory; receptor; septum; synapse.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Hausott B., Schlick B., Vallant N., Dorn R., Klimaschewski L. Promotion of neurite outgrowth by fibroblast growth factor receptor 1 overexpression and lysosomal inhibition of receptor degradation in pheochromocytoma cells and adult sensory neurons. Neuroscience. 2008;153:461–473. doi: 10.1016/j.neuroscience.2008.01.083. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

