Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice
- PMID: 32586265
- PMCID: PMC7318443
- DOI: 10.1186/s12263-020-00671-3
Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice
Abstract
Background: Adiponectin and resistin are typically secreted by the adipose tissue and are abnormally expressed in obesity. However, the underlying influential factors and mechanisms are to be elucidated. It is well known that the expression of genes is regulated by epigenetics while gut microbiota participates in epigenetic processes through its metabolites such as folate, biotin, and short-chain fatty acids (SCFAs). Therefore, we supposed that alteration of gut microbiota might affect the transcriptional expression of adiponectin and resistin through epigenetic regulation in obesity.
Methods: C57BL/6J mice were fed either a high-fat diet (34.9% fat by wt., 60% kcal) or a normal-fat diet (4.3% fat by wt., 10% kcal) for 16 weeks, with ampicillin and neomycin delivered via drinking water to interfere with gut microbiota development. Fecal microbiota was analyzed by 16S rRNA high-throughput sequencing. The mRNA expression levels of genes were measured by real-time quantitative RT-PCR. SCFA contents in feces were examined using gas chromatography.
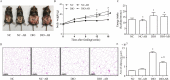
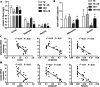
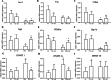
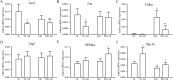
Results: Alteration of the gut microbiota induced by antibiotic use, characterized by a dramatic reduction of the phylum Firmicutes and Actinobacteria and an increase of Proteobacteria with reductions of genera including Lactobacillus, norank_f_Bacteroidales_S24-7_group, Alistipes, Desulfovibrio, Helicobacter, etc., and increases in Bacteroides, Enterobacter, Klebsiella, inhibited the body weight gain in mice fed the high-fat diet instead of the normal-fat diet. The mRNA expression of adiponectin and resistin was upregulated by antibiotic use in mice fed the high-fat diet, accompanied by increased expression of fat oxidation and thermogenesis-related genes (PPAR-α, Pgc-1α, and Atgl) in the fat and/or liver, whereas no change in the expression of adiponectin and resistin was found in mice fed the normal-fat diet. Furthermore, antibiotic use reduced DNA methylation fractions of the adiponectin and resistin promoters and downregulated the expression of DNA methyltransferase 1 and 3a (DNMT1 and DNMT3a) with the high-fat diet feeding.
Conclusion: Alteration of gut microbiota induced by antibiotic use may affect the expression of adiponectin and resistin in mice fed the high-fat diet by modifying promoter DNA methylation, thus leading to increased fatty acid oxidation and less body weight gain.
Keywords: Adipokines; Antibiotics; DNA methylation; Gut microbiota; Obesity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Suppression of high-fat-diet-induced obesity in mice by dietary folic acid supplementation is linked to changes in gut microbiota.Eur J Nutr. 2022 Jun;61(4):2015-2031. doi: 10.1007/s00394-021-02769-9. Epub 2022 Jan 6. Eur J Nutr. 2022. PMID: 34993642
-
Effects of SCFA on the DNA methylation pattern of adiponectin and resistin in high-fat-diet-induced obese male mice.Br J Nutr. 2018 Aug;120(4):385-392. doi: 10.1017/S0007114518001526. Epub 2018 Jun 21. Br J Nutr. 2018. PMID: 29925443
-
Dietary fat and gut microbiota interactions determine diet-induced obesity in mice.Mol Metab. 2016 Oct 13;5(12):1162-1174. doi: 10.1016/j.molmet.2016.10.001. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900259 Free PMC article.
-
The Epigenetic Connection Between the Gut Microbiome in Obesity and Diabetes.Front Genet. 2020 Jan 15;10:1329. doi: 10.3389/fgene.2019.01329. eCollection 2019. Front Genet. 2020. PMID: 32010189 Free PMC article. Review.
-
Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies.Foods. 2021 Feb 2;10(2):299. doi: 10.3390/foods10020299. Foods. 2021. PMID: 33540692 Free PMC article. Review.
Cited by
-
Electroacupuncture Combined With Diet Treatment Has a Therapeutic Effect on Perimenopausal Patients With Abdominal Obesity by Improving the Community Structure of Intestinal Flora.Front Physiol. 2021 Nov 25;12:708588. doi: 10.3389/fphys.2021.708588. eCollection 2021. Front Physiol. 2021. PMID: 34899365 Free PMC article.
-
Urbanization and Unfavorable Changes in Metabolic Profiles: A Prospective Cohort Study of Indonesian Young Adults.Nutrients. 2022 Aug 14;14(16):3326. doi: 10.3390/nu14163326. Nutrients. 2022. PMID: 36014832 Free PMC article.
-
Inflammatory Manifestations Associated With Gut Dysbiosis in Alzheimer's Disease.Int J Alzheimers Dis. 2024 Sep 20;2024:9741811. doi: 10.1155/2024/9741811. eCollection 2024. Int J Alzheimers Dis. 2024. PMID: 39346576 Free PMC article.
-
Suppression of high-fat-diet-induced obesity in mice by dietary folic acid supplementation is linked to changes in gut microbiota.Eur J Nutr. 2022 Jun;61(4):2015-2031. doi: 10.1007/s00394-021-02769-9. Epub 2022 Jan 6. Eur J Nutr. 2022. PMID: 34993642
-
Faecal Microbiota transplantation affects liver DNA methylation in Non-alcoholic fatty liver disease: a multi-omics approach.Gut Microbes. 2023 Dec 31;15(1):2223330. doi: 10.1080/19490976.2023.2223330. Gut Microbes. 2023. PMID: 37317027 Free PMC article.
References
Grants and funding
- 81670775/National Natural Science Foundation of China
- 2014-07, 2015-E2/Nutricia Research Foundation
- 2017-bjsekyjs/Research Funds of Profession Quota Budget from Beijing Municipal Science and Technology Commission
- Discipline Backbone 2009-3-40/Funds for High-Level Technical Talents in the Beijing Health System
LinkOut - more resources
Full Text Sources

