Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial
- PMID: 32586583
- PMCID: PMC8064404
- DOI: 10.1016/j.jacc.2020.04.065
Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial
Abstract
Background: The CABANA (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation) trial randomized 2,204 patients with atrial fibrillation (AF) to catheter ablation or drug therapy. Analysis by intention-to-treat showed a nonsignificant 14% relative reduction in the primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
Objectives: The purpose of this study was to assess recurrence of AF in the CABANA trial.
Methods: The authors prospectively studied CABANA patients using a proprietary electrocardiogram recording monitor for symptom-activated and 24-h AF auto detection. The AF recurrence endpoint was any post-90-day blanking atrial tachyarrhythmias lasting 30 s or longer. Biannual 96-h Holter monitoring was used to assess AF burden. Patients who used the CABANA monitors and provided 90-day post-blanking recordings qualified for this analysis (n = 1,240; 56% of CABANA population). Treatment comparisons were performed using a modified intention-to-treat approach.
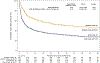
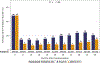
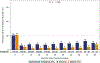
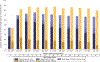
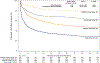
Results: Median age of the 1,240 patients was 68 years, 34.4% were women, and AF was paroxysmal in 43.0%. Over 60 months of follow-up, first recurrence of any symptomatic or asymptomatic AF (hazard ratio: 0.52; 95% confidence interval: 0.45 to 0.60; p < 0.001) or first symptomatic-only AF (hazard ratio: 0.49; 95% confidence interval: 0.39 to 0.61; p < 0.001) were both significantly reduced in the catheter ablation group. Baseline Holter AF burden in both treatment groups was 48%. At 12 months, AF burden in ablation patients averaged 6.3%, and in drug-therapy patients, 14.4%. AF burden was significantly less in catheter ablation compared with drug-therapy patients across the 5-year follow-up (p < 0.001). These findings were not sensitive to the baseline pattern of AF.
Conclusions: Catheter ablation was effective in reducing recurrence of any AF by 48% and symptomatic AF by 51% compared with drug therapy over 5 years of follow-up. Furthermore, AF burden was also significantly reduced in catheter ablation patients, regardless of their baseline AF type. (Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial [CABANA]; NCT00911508).
Keywords: antiarrhythmic drug therapy; atrial fibrillation; catheter ablation; long-standing persistent atrial fibrillation; paroxysmal atrial fibrillation; persistent atrial fibrillation; pulmonary vein isolation.
Copyright © 2020 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Reporting AF Recurrence After Catheter Ablation: The Burden Is on Us to Get it Right.J Am Coll Cardiol. 2020 Jun 30;75(25):3119-3121. doi: 10.1016/j.jacc.2020.04.066. J Am Coll Cardiol. 2020. PMID: 32586584 No abstract available.
-
Comparison of left atrial and left atrial appendage mechanics in the recurrence of atrial fibrillation after radiofrequency catheter ablation.Echocardiography. 2023 Oct;40(10):1048-1057. doi: 10.1111/echo.15670. Epub 2023 Aug 7. Echocardiography. 2023. PMID: 37548034
References
-
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95(3):572–6. - PubMed
-
- Calkins H, Kuck K, Cappato R et al. 2012 HRS/EHRA/ECAS/ACC/AHA/ APHRS/STS/ESC expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendation for patient selection, procedural techniques, patient management, and follow up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm 2012;9:632–6. - PubMed
-
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293(21):2634–40. - PubMed
-
- Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006;48(11):2340–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

