Mitochondrial damage and senescence phenotype of cells derived from a novel frataxin G127V point mutation mouse model of Friedreich's ataxia
- PMID: 32586831
- PMCID: PMC7406325
- DOI: 10.1242/dmm.045229
Mitochondrial damage and senescence phenotype of cells derived from a novel frataxin G127V point mutation mouse model of Friedreich's ataxia
Abstract
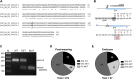
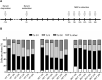
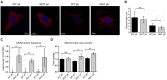
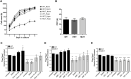
Friedreich's ataxia (FRDA) is an autosomal recessive neurodegenerative disease caused by reduced expression of the mitochondrial protein frataxin (FXN). Most FRDA patients are homozygous for large expansions of GAA repeat sequences in intron 1 of FXN, whereas a fraction of patients are compound heterozygotes, with a missense or nonsense mutation in one FXN allele and expanded GAAs in the other. A prevalent missense mutation among FRDA patients changes a glycine at position 130 to valine (G130V). Herein, we report generation of the first mouse model harboring an Fxn point mutation. Changing the evolutionarily conserved glycine 127 in mouse Fxn to valine results in a failure-to-thrive phenotype in homozygous animals and a substantially reduced number of offspring. Like G130V in FRDA, the G127V mutation results in a dramatic decrease of Fxn protein without affecting transcript synthesis or splicing. FxnG127V mouse embryonic fibroblasts exhibit significantly reduced proliferation and increased cell senescence. These defects are evident in early passage cells and are exacerbated at later passages. Furthermore, increased frequency of mitochondrial DNA lesions and fragmentation are accompanied by marked amplification of mitochondrial DNA in FxnG127V cells. Bioenergetics analyses demonstrate higher sensitivity and reduced cellular respiration of FxnG127V cells upon alteration of fatty acid availability. Importantly, substitution of FxnWT with FxnG127V is compatible with life, and cellular proliferation defects can be rescued by mitigation of oxidative stress via hypoxia or induction of the NRF2 pathway. We propose FxnG127V cells as a simple and robust model for testing therapeutic approaches for FRDA.
Keywords: Frataxin; Friedreich's ataxia; Mitochondria; Oxidative stress; Point mutation; Senescence.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

