Dynamic human MutSα-MutLα complexes compact mismatched DNA
- PMID: 32586954
- PMCID: PMC7368325
- DOI: 10.1073/pnas.1918519117
Dynamic human MutSα-MutLα complexes compact mismatched DNA
Abstract
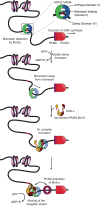
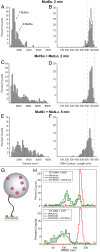
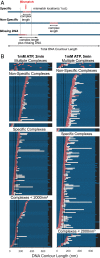
DNA mismatch repair (MMR) corrects errors that occur during DNA replication. In humans, mutations in the proteins MutSα and MutLα that initiate MMR cause Lynch syndrome, the most common hereditary cancer. MutSα surveilles the DNA, and upon recognition of a replication error it undergoes adenosine triphosphate-dependent conformational changes and recruits MutLα. Subsequently, proliferating cell nuclear antigen (PCNA) activates MutLα to nick the error-containing strand to allow excision and resynthesis. The structure-function properties of these obligate MutSα-MutLα complexes remain mostly unexplored in higher eukaryotes, and models are predominately based on studies of prokaryotic proteins. Here, we utilize atomic force microscopy (AFM) coupled with other methods to reveal time- and concentration-dependent stoichiometries and conformations of assembling human MutSα-MutLα-DNA complexes. We find that they assemble into multimeric complexes comprising three to eight proteins around a mismatch on DNA. On the timescale of a few minutes, these complexes rearrange, folding and compacting the DNA. These observations contrast with dominant models of MMR initiation that envision diffusive MutS-MutL complexes that move away from the mismatch. Our results suggest MutSα localizes MutLα near the mismatch and promotes DNA configurations that could enhance MMR efficiency by facilitating MutLα nicking the DNA at multiple sites around the mismatch. In addition, such complexes may also protect the mismatch region from nucleosome reassembly until repair occurs, and they could potentially remodel adjacent nucleosomes.
Keywords: AFM; DNA repair; DREEM; MutL; MutS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

