Inhibition of SARS-CoV-2 by type I and type III interferons
- PMID: 32587093
- PMCID: PMC7549028
- DOI: 10.1074/jbc.AC120.013788
Inhibition of SARS-CoV-2 by type I and type III interferons
Abstract
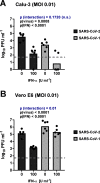
The recently emerged severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of the devastating COVID-19 lung disease pandemic. Here, we tested the inhibitory activities of the antiviral interferons of type I (IFN-α) and type III (IFN-λ) against SARS-CoV-2 and compared them with those against SARS-CoV-1, which emerged in 2003. Using two mammalian epithelial cell lines (human Calu-3 and simian Vero E6), we found that both IFNs dose-dependently inhibit SARS-CoV-2. In contrast, SARS-CoV-1 was restricted only by IFN-α in these cell lines. SARS-CoV-2 generally exhibited a broader IFN sensitivity than SARS-CoV-1. Moreover, ruxolitinib, an inhibitor of IFN-triggered Janus kinase/signal transducer and activator of transcription signaling, boosted SARS-CoV-2 replication in the IFN-competent Calu-3 cells. We conclude that SARS-CoV-2 is sensitive to exogenously added IFNs. This finding suggests that type I and especially the less adverse effect-prone type III IFN are good candidates for the management of COVID-19.
Keywords: COVID-19; SARS–CoV-2; antiviral agent; cytokine action; infection; innate immunity; interferon; interferon-alpha/beta; interferon-lambda; ruxolitinib; virology; virus.
© 2020 Felgenhauer et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Similar articles
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
Type I Interferon Susceptibility Distinguishes SARS-CoV-2 from SARS-CoV.J Virol. 2020 Nov 9;94(23):e01410-20. doi: 10.1128/JVI.01410-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32938761 Free PMC article.
-
Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19.Cell Host Microbe. 2020 Jun 10;27(6):870-878. doi: 10.1016/j.chom.2020.05.008. Epub 2020 May 27. Cell Host Microbe. 2020. PMID: 32464097 Free PMC article. Review.
-
Antiviral activities of type I interferons to SARS-CoV-2 infection.Antiviral Res. 2020 Jul;179:104811. doi: 10.1016/j.antiviral.2020.104811. Epub 2020 Apr 29. Antiviral Res. 2020. PMID: 32360182 Free PMC article.
-
Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection.Eur J Immunol. 2021 May;51(5):1071-1075. doi: 10.1002/eji.202149173. Epub 2021 Mar 22. Eur J Immunol. 2021. PMID: 33675065 Free PMC article. Review.
Cited by
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
Interferon Resistance of Emerging SARS-CoV-2 Variants.bioRxiv [Preprint]. 2021 Dec 10:2021.03.20.436257. doi: 10.1101/2021.03.20.436257. bioRxiv. 2021. Update in: Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2203760119. doi: 10.1073/pnas.2203760119. PMID: 33758840 Free PMC article. Updated. Preprint.
-
SARS-CoV-2: Recent Variants and Clinical Efficacy of Antibody-Based Therapy.Front Cell Infect Microbiol. 2022 Feb 14;12:839170. doi: 10.3389/fcimb.2022.839170. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35237535 Free PMC article. Review.
-
Type III interferon exerts thymic stromal lymphopoietin in mediating adaptive antiviral immune response.Front Immunol. 2023 Sep 22;14:1250541. doi: 10.3389/fimmu.2023.1250541. eCollection 2023. Front Immunol. 2023. PMID: 37809098 Free PMC article. Review.
-
Interferon at the crossroads of SARS-CoV-2 infection and COVID-19 disease.J Biol Chem. 2023 Aug;299(8):104960. doi: 10.1016/j.jbc.2023.104960. Epub 2023 Jun 24. J Biol Chem. 2023. PMID: 37364688 Free PMC article. Review.
References
-
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., et al. (2020) Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 27, 325–328 10.1016/j.chom.2020.02.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

