Contribution of Monocarboxylate Transporter 6 to the Pharmacokinetics and Pharmacodynamics of Bumetanide in Mice
- PMID: 32587098
- PMCID: PMC7469248
- DOI: 10.1124/dmd.120.000068
Contribution of Monocarboxylate Transporter 6 to the Pharmacokinetics and Pharmacodynamics of Bumetanide in Mice
Abstract
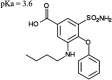
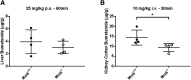
Bumetanide, a sulfamyl loop diuretic, is used for the treatment of edema in association with congestive heart failure. Being a polar, anionic compound at physiologic pH, bumetanide uptake and efflux into different tissues is largely transporter-mediated. Of note, organic anion transporters (SLC22A) have been extensively studied in terms of their importance in transporting bumetanide to its primary site of action in the kidney. The contribution of one of the less-studied bumetanide transporters, monocarboxylate transporter 6 (MCT6; SLC16A5), to bumetanide pharmacokinetics (PK) and pharmacodynamics (PD) has yet to be characterized. The affinity of bumetanide for murine Mct6 was evaluated using Mct6-transfected Xenopus laevis oocytes. Furthermore, bumetanide was intravenously and orally administered to wild-type mice (Mct6+/+) and homozygous Mct6 knockout mice (Mct6-/-) to elucidate the contribution of Mct6 to bumetanide PK/PD in vivo. We demonstrated that murine Mct6 transports bumetanide at a similar affinity compared with human MCT6 (78 and 84 μM, respectively, at pH 7.4). After bumetanide administration, there were no significant differences in plasma PK. Additionally, diuresis was significantly decreased by ∼55% after intravenous bumetanide administration in Mct6-/- mice. Kidney cortex concentrations of bumetanide were decreased, suggesting decreased Mct6-mediated bumetanide transport to its site of action in the kidney. Overall, these results suggest that Mct6 does not play a major role in the plasma PK of bumetanide in mice; however, it significantly contributes to bumetanide's pharmacodynamics due to changes in kidney concentrations. SIGNIFICANCE STATEMENT: Previous evidence suggested that MCT6 transports bumetanide in vitro; however, no studies to date have evaluated the in vivo contribution of this transporter. In vitro studies indicated that mouse and human MCT6 transport bumetanide with similar affinities. Using Mct6 knockout mice, we demonstrated that murine Mct6 does not play a major role in the plasma pharmacokinetics of bumetanide; however, the pharmacodynamic effect of diuresis was attenuated in the knockout mice, likely because of the decreased bumetanide concentrations in the kidney.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures





Similar articles
-
Quercetin, Morin, Luteolin, and Phloretin Are Dietary Flavonoid Inhibitors of Monocarboxylate Transporter 6.Mol Pharm. 2017 Sep 5;14(9):2930-2936. doi: 10.1021/acs.molpharmaceut.7b00264. Epub 2017 May 31. Mol Pharm. 2017. PMID: 28513167 Free PMC article.
-
Functional characterization of human monocarboxylate transporter 6 (SLC16A5).Drug Metab Dispos. 2005 Dec;33(12):1845-51. doi: 10.1124/dmd.105.005264. Epub 2005 Sep 20. Drug Metab Dispos. 2005. PMID: 16174808
-
Monocarboxylate Transporter 6-Mediated Interactions with Prostaglandin F2α: In Vitro and In Vivo Evidence Utilizing a Knockout Mouse Model.Pharmaceutics. 2020 Feb 26;12(3):201. doi: 10.3390/pharmaceutics12030201. Pharmaceutics. 2020. PMID: 32110957 Free PMC article.
-
Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease.Pharmacol Rev. 2020 Apr;72(2):466-485. doi: 10.1124/pr.119.018762. Pharmacol Rev. 2020. PMID: 32144120 Free PMC article. Review.
-
CNS pharmacology of NKCC1 inhibitors.Neuropharmacology. 2022 Mar 1;205:108910. doi: 10.1016/j.neuropharm.2021.108910. Epub 2021 Dec 6. Neuropharmacology. 2022. PMID: 34883135 Review.
Cited by
-
Efficacy of bumetanide tablets combined with valsartan in the treatment of elderly patients with chronic glomerulonephritis and its effects on renal function and hemodynamics.Am J Transl Res. 2023 May 15;15(5):3424-3432. eCollection 2023. Am J Transl Res. 2023. PMID: 37303676 Free PMC article.
-
Role of Monovalent Ions in the NKCC1 Inhibition Mechanism Revealed through Molecular Simulations.Int J Mol Sci. 2022 Dec 6;23(23):15439. doi: 10.3390/ijms232315439. Int J Mol Sci. 2022. PMID: 36499764 Free PMC article.
References
-
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. (2009) Cation-chloride cotransporters and neuronal function. Neuron 61:820–838. - PubMed
-
- Brater DC, Day B, Burdette A, Anderson S. (1984) Bumetanide and furosemide in heart failure. Kidney Int 26:183–189. - PubMed
-
- Cook JA, Smith DE, Cornish LA, Tankanow RM, Nicklas JM, Hyneck ML. (1988) Kinetics, dynamics, and bioavailability of bumetanide in healthy subjects and patients with congestive heart failure. Clin Pharmacol Ther 44:487–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

