Pre-innervated tissue-engineered muscle promotes a pro-regenerative microenvironment following volumetric muscle loss
- PMID: 32587337
- PMCID: PMC7316777
- DOI: 10.1038/s42003-020-1056-4
Pre-innervated tissue-engineered muscle promotes a pro-regenerative microenvironment following volumetric muscle loss
Abstract
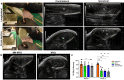
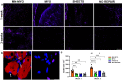
Volumetric muscle loss (VML) is the traumatic or surgical loss of skeletal muscle beyond the inherent regenerative capacity of the body, generally leading to severe functional deficit. Formation of appropriate somato-motor innervations remains one of the biggest challenges for both autologous grafts as well as tissue-engineered muscle constructs. We aim to address this challenge by developing pre-innervated tissue-engineered muscle comprised of long aligned networks of spinal motor neurons and skeletal myocytes on aligned nanofibrous scaffolds. Motor neurons led to enhanced differentiation and maturation of skeletal myocytes in vitro. These pre-innervated tissue-engineered muscle constructs when implanted in a rat VML model significantly increased satellite cell density, neuromuscular junction maintenance, graft revascularization, and muscle volume over three weeks as compared to myocyte-only constructs and nanofiber scaffolds alone. These pro-regenerative effects may enhance functional neuromuscular regeneration following VML, thereby improving the levels of functional recovery following these devastating injuries.
Conflict of interest statement
D.K.C is a co-founder of Axonova Medical, LLC, and INNERVACE, Inc, which are University of Pennsylvania spin-out companies focused on translation of advanced regenerative therapies to treat nervous system disorders. U.S. Provisional Patent App. 62/758,203 (D.K.C., S.D.) has been filed related to the technology of fabricating innervated tissue-engineered constructs. No other author has declared a potential conflict of interest.
Figures








References
-
- Oberpenning, F., Meng, J., Yoo, J. J. & Atala, A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 10.1038/6146 (1999). - PubMed
-
- Morimoto, Y., Kato-Negishi, M., Onoe, H. & Takeuchi, S. Three-dimensional neuron-muscle constructs with neuromuscular junctions. Biomaterials10.1016/j.biomaterials.2013.08.062 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

