TET1 Deficiency Impairs Morphogen-free Differentiation of Human Embryonic Stem Cells to Neuroectoderm
- PMID: 32587369
- PMCID: PMC7316867
- DOI: 10.1038/s41598-020-67143-x
TET1 Deficiency Impairs Morphogen-free Differentiation of Human Embryonic Stem Cells to Neuroectoderm
Abstract
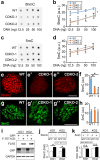
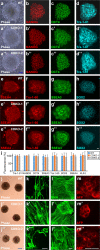
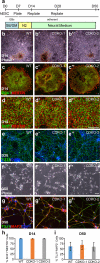
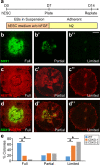
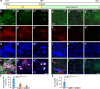
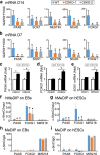
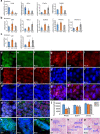
The TET family of 5-methylcytosine (5mC) dioxygenases plays critical roles in development by modifying DNA methylation. Using CRISPR, we inactivated the TET1 gene in H9 human embryonic stem cells (hESCs). Mutant H9 hESCs remained pluripotent, even though the level of hydroxymethylcytosine (5hmC) decreased to 30% of that in wild-type cells. Neural differentiation induced by dual SMAD inhibitors was not significantly affected by loss of TET1 activity. However, in a morphogen-free condition, TET1 deficiency significantly reduced the generation of NESTIN+SOX1+ neuroectoderm cells from 70% in wild-type cells to 20% in mutant cells. This was accompanied by a 20-fold reduction in the expression level of PAX6 and a significant decrease in the amount of 5hmC on the PAX6 promoter. Overexpression of the TET1 catalytic domain in TET1-deficient hESCs significantly increased 5hmC levels and elevated PAX6 expression during differentiation. Consistent with these in vitro data, PAX6 expression was significantly decreased in teratomas formed by TET1-deficient hESCs. However, TET1 deficiency did not prevent the formation of neural tube-like structures in teratomas. Our results suggest that TET1 deficiency impairs the intrinsic ability of hESCs to differentiate to neuroectoderm, presumably by decreasing the expression of PAX6, a key regulator in the development of human neuroectoderm.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
TET proteins safeguard bivalent promoters from de novo methylation in human embryonic stem cells.Nat Genet. 2018 Jan;50(1):83-95. doi: 10.1038/s41588-017-0002-y. Epub 2017 Dec 4. Nat Genet. 2018. PMID: 29203910 Free PMC article.
-
Distinct and overlapping control of 5-methylcytosine and 5-hydroxymethylcytosine by the TET proteins in human cancer cells.Genome Biol. 2014 Jun 23;15(6):R81. doi: 10.1186/gb-2014-15-6-r81. Genome Biol. 2014. PMID: 24958354 Free PMC article.
-
Epigenetic regulation of human adipose-derived stem cells differentiation.Mol Cell Biochem. 2015 Dec;410(1-2):111-20. doi: 10.1007/s11010-015-2543-7. Epub 2015 Aug 26. Mol Cell Biochem. 2015. PMID: 26307369
-
Role of ten-eleven translocation proteins and 5-hydroxymethylcytosine in hepatocellular carcinoma.Cell Prolif. 2019 Jul;52(4):e12626. doi: 10.1111/cpr.12626. Epub 2019 Apr 29. Cell Prolif. 2019. PMID: 31033072 Free PMC article. Review.
-
Multiple Functions of Ten-eleven Translocation 1 during Tumorigenesis.Chin Med J (Engl). 2016 Jul 20;129(14):1744-51. doi: 10.4103/0366-6999.185873. Chin Med J (Engl). 2016. PMID: 27411465 Free PMC article. Review.
Cited by
-
Epigenetic Regulation of TET1-SP1 During Spermatogonia Self-Renewal and Proliferation.Front Physiol. 2022 Feb 11;13:843825. doi: 10.3389/fphys.2022.843825. eCollection 2022. Front Physiol. 2022. PMID: 35222097 Free PMC article.
-
Multi-Target Neural Differentiation (MTND) Therapeutic Cocktail to Suppress Brain Tumor.Int J Mol Sci. 2023 Aug 2;24(15):12329. doi: 10.3390/ijms241512329. Int J Mol Sci. 2023. PMID: 37569705 Free PMC article.
-
Structure and Function of TET Enzymes.Adv Exp Med Biol. 2022;1389:239-267. doi: 10.1007/978-3-031-11454-0_10. Adv Exp Med Biol. 2022. PMID: 36350513
-
Epigenetic Reprogramming in Mice and Humans: From Fertilization to Primordial Germ Cell Development.Cells. 2023 Jul 17;12(14):1874. doi: 10.3390/cells12141874. Cells. 2023. PMID: 37508536 Free PMC article. Review.
-
New Insights into TETs in Psychiatric Disorders.Int J Mol Sci. 2022 Apr 28;23(9):4909. doi: 10.3390/ijms23094909. Int J Mol Sci. 2022. PMID: 35563298 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

