Amylin and beta amyloid proteins interact to form amorphous heterocomplexes with enhanced toxicity in neuronal cells
- PMID: 32587390
- PMCID: PMC7316712
- DOI: 10.1038/s41598-020-66602-9
Amylin and beta amyloid proteins interact to form amorphous heterocomplexes with enhanced toxicity in neuronal cells
Abstract
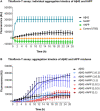
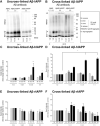
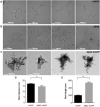
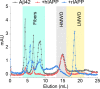
Human pancreatic islet amyloid polypeptide (hIAPP) and beta amyloid (Aβ) can accumulate in Type 2 diabetes (T2D) and Alzheimer's disease (AD) brains and evidence suggests that interaction between the two amyloidogenic proteins can lead to the formation of heterocomplex aggregates. However, the structure and consequences of the formation of these complexes remains to be determined. The main objective of this study was to characterise the different types and morphology of Aβ-hIAPP heterocomplexes and determine if formation of such complexes exacerbate neurotoxicity. We demonstrate that hIAPP promotes Aβ oligomerization and formation of small oligomer and large aggregate heterocomplexes. Co-oligomerized Aβ42-hIAPP mixtures displayed distinct amorphous structures and a 3-fold increase in neuronal cell death as compared to Aβ and hIAPP alone. However, in contrast to hIAPP, non-amyloidogenic rat amylin (rIAPP) reduced oligomer Aβ-mediated neuronal cell death. rIAPP exhibited reductions in Aβ induced neuronal cell death that was independent of its ability to interact with Aβ and form heterocomplexes; suggesting mediation by other pathways. Our findings reveal distinct effects of IAPP peptides in modulating Aβ aggregation and toxicity and provide new insight into the potential pathogenic effects of Aβ-IAPP hetero-oligomerization and development of IAPP based therapies for AD and T2D.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Ninomiya T. Diabetes mellitus and dementia. Curr Diabetes Rep. 2014;14:1–9. - PubMed
-
- Ott A, et al. Association between diabetes mellitus and dementia: the Rotterdam study. Diebetologia. 1996;39:1392–1397. - PubMed
-
- Verdile G, Fuller SJ, Martins R. The role of Type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015;84:22–38. - PubMed
-
- Bharadwaj P, et al. The link between Type 2 diabetes and neurodegeneration: roles for amyloid-β, amylin, and tau proteins. J Alzheimer Dis. 2017;59:421–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

