An Economical Route to Lamivudine Featuring a Novel Strategy for Stereospecific Assembly
- PMID: 32587454
- PMCID: PMC7309434
- DOI: 10.1021/acs.oprd.0c00083
An Economical Route to Lamivudine Featuring a Novel Strategy for Stereospecific Assembly
Abstract
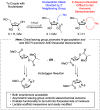
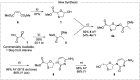
An economical synthesis of lamivudine was developed by employing a new method to establish the stereochemistry about the heterocyclic oxathiolane ring. Toward this end, an inexpensive and readily accessible lactic acid derivative served the dual purpose of activating the carbohydrate's anomeric center for N-glycosylation and transferring stereochemical information to the substrate simultaneously. Both enantiomers of the lactic acid derivative are available, and either β-enantiomer in this challenging class of 2'-deoxynucleoside active pharmaceutical ingredients can be formed.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Panos Z.; Fox J.; Prabhu V.. HIV Market Report, issue (10), , September 2019. http://clintonhealthaccess.org/wp-content/uploads/2019/12/2019-HIV-Marke... (accessed 2020-02-06).
- World Health Organization . HIV country profiles. https://www.who.int/hiv/data/profiles/en/ (accessed 2020-04-02).
- World Health Organization , Data on the size of the HIV/AIDS epidemic, https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/data-o... (accessed 2020-04-02).
- World Atlas . Countries with the Highest Rates of HIV/AIDS. https://www.worldatlas.com/articles/countries-with-the-highest-rates-of-... (accessed 2020-04-02).
-
-
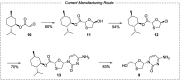
The commercial route was confirmed through conversation with manufacturers and by analysis of high-volume transaction records of API intermediates moving through the market (menthol glyoxylate and menthol ester of hydroxyl or cytosinyl oxathiolane).
- Goodyear M. D.; Dwyer P. O.; Hill M. L.; Whitehead A. J.; Hornby R.; Hallet P.. Process for the diastereoselective synthesis of nucleoside analogues. US 6051709, 2000.
- Goodyear M. D.; Hill M. L.; West J. P.; Whitehead A. J. Practical enantioselective synthesis of lamivudine (3TC) via a dynamic kinetic resolution. Tetrahedron Lett. 2005, 46, 8535–8538. 10.1016/j.tetlet.2005.10.002. - DOI
-
-
- Watanabe K. A.; Hollenberg D. H.; Fox J. J. Nucleosides. 85. On mechanisms of nucleoside synthesis by condensation reactions. J. Carbohydr., Nucleosides, Nucleotides 1974, 1, 1–37.
- Wilson L. J.; Hager M. W.; El-Kattan Y. A.; Liotta D. C. Nitrogen lycosylation reactions involving pyrimidine and purine nucleoside bases with furanoside sugars. Synthesis 1995, 1995, 1465–1479. 10.1055/s-1995-4142. - DOI
- Mukaiyama T.; Hirano N.; Nishida M.; Uchiro H. Catalytic stereoselective synthesis of pyrimidine 2-deoxyribonucleosides. Chem. Lett. 1996, 25, 99–100. 10.1246/cl.1996.99. - DOI
- McLaughlin M.; Kong J.; Belyk K. M.; Chen B.; Gibson A. W.; Keen S. P.; Lieberman D. R.; Milczek E. M.; Moore J. C.; Murray D.; Qi J.; Peng F.; Reamer R. A.; Song Z. J.; Tan L.; Wang L.; Williams M. J. Enantioselective synthesis of 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) via enzymatic desymmetrization. Org. Lett. 2017, 19, 926–929. 10.1021/acs.orglett.7b00091. - DOI - PubMed
-
- Jeong L. S.; Schinazi R. F.; Beach W. J.; Kim H. O.; Nampalli S.; Shanmuganathan K.; Alves A. J.; McMillan A.; Chu C. K.; Mathis R. Asymmetric synthesis and biological evaluation of β-l-(2R,5S)- and α-l-(2R,5R)-1,3-oxathiolane-pyrimidine and -purine nucleosides as potential anti-HIV agents. J. Med. Chem. 1993, 36, 181–195. 10.1021/jm00054a001. - DOI - PubMed
- Hu L.; Schaufelberger F.; Zhang Y.; Ramström O. Efficient asymmetric synthesis of lamivudine via enzymatic dynamic kinetic resolution. Chem. Commun. 2013, 49, 10376–10378. 10.1039/c3cc45551c. - DOI - PubMed
-
- Hoong L. K.; Strange L. E.; Liotta D. C.; Koszalka G. W.; Burns C. L.; Schinazi R. F. Enzyme-mediated enantioselective preparation of pure enantiomers of the antiviral agent 2′,3′-dideoxy-5-fluoro-3′-thiacytidine (FTC) and related compounds. J. Org. Chem. 1992, 57, 5563–5565. 10.1021/jo00047a004. - DOI
- Mahmoudian M.; Baines B. S.; Drake C. S.; Hale R. S.; Jones P.; Piercey J. E.; Montgomery D. S.; Purvis I. J.; Storer R.; Dawson M. J.; Lawrence G. C. Enzymatic production of optically pure (2′R-cis)-2′-deoxy-3′-thiacytidine (3TC, Lamivudine): A potent anti-HIV agent. Enzyme Microb. Technol. 1993, 15, 749–755. 10.1016/0141-0229(93)90005-M. - DOI - PubMed
- Milton J.; Brand S.; Jones M. F.; Rayner C. M. Enzymatic Resolution of α-acetoxysulfides: A new approach to the synthesis of homochiral S, O-Acetals. Tetrahedron: Asymmetry 1995, 6, 1903–1906. 10.1016/0957-4166(95)00248-N. - DOI
- Milton J.; Brand S.; Jones M. F.; Rayner C. M. Enantioselective enzymatic synthesis of the anti-viral agent lamivudine (3TC). Tetrahedron Lett. 1995, 36, 6961–6964. 10.1016/0040-4039(95)01380-Z. - DOI
- Cousins R. P. C.; Mahmoudian M.; Youds P. M. Enantioselective enzymatic synthesis of the anti-viral agent lamivudine (3TC). Tetrahedron: Asymmetry 1995, 6, 393–396. 10.1016/0957-4166(95)00022-H. - DOI
- Jin H.; Siddiqui A.; Evans C. A.; Tse H. L. A.; Mansour T. S.; Goodyear M. D.; Ravenscroft P.; Beels C. D. Diastereoselective synthesis of the potent antiviral agent (−)-2′-deoxy-3′-thiacytidine and its enantiomer. J. Org. Chem. 1995, 60, 2621–2623. 10.1021/jo00113a050. - DOI
- Li J.-Z.; Gao L.-X.; Ding M.-X. The chemical resolution of racemic cis-2-hydroxymethyl-5-(cytosine-1′-yl)-1,3-oxathiolane (BCH-189)—One direct method to obtain lamivudine as anti-HIV and anti-HBV agent. Synth. Commun. 2002, 32, 2355–2359. 10.1081/SCC-120006006. - DOI
- Roy B. N.; Singh G. P.; Srivastava D.; Jadhav H. S.; Saini M. B.; Aher U. P. A Novel Method for Large-Scale Synthesis of Lamivudine through Cocrystal Formation of Racemic Lamivudine with (S)-(−)-1,1′-Bi(2-naphthol) [(S)-(BINOL)]. Org. Process Res. Dev. 2009, 13, 450–455. 10.1021/op800228h. - DOI
- Reddy B. P.; Reddy K. R.; Reddy R. R.; Reddy D. M.; Srinivas A. S.. Optical resolution of substituted 1,3-oxathiolane nucleosides. US 20110245497, 2011.
LinkOut - more resources
Full Text Sources
