Antidepressant Prescribing and Suicide/Self-Harm by Young Australians: Regulatory Warnings, Contradictory Advice, and Long-Term Trends
- PMID: 32587531
- PMCID: PMC7299202
- DOI: 10.3389/fpsyt.2020.00478
Antidepressant Prescribing and Suicide/Self-Harm by Young Australians: Regulatory Warnings, Contradictory Advice, and Long-Term Trends
Abstract
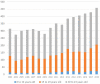
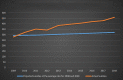
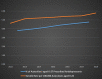
In 2004, the US Food and Drug Administration (FDA) controversially issued a black box warning that antidepressants were associated with an increased risk of suicidal thoughts and behaviours in people aged under 18 years. In 2007, the warning was expanded to include young adults aged under 25 years. In 2005, the Australian Therapeutic Goods Administration responded to the FDA warning by requiring Product and Consumer Information leaflets to be updated to reflect the risk. However, there was considerable debate, and at times emotive backlash, in academic journals and the international media. Prominent US and Australian mental health organisations and psychiatrists challenged the FDA warning. They argued that, on balance, antidepressant use was likely to reduce the risk of suicide. Several ecological studies were cited misleadingly as evidence that decreasing antidepressant use increases suicide risk. From 2008 to 2018, Australian per-capita child, adolescent and young adult antidepressant dispensing (0-27 years of age) and suicide (0-24 years) rates have increased approximately 66% and 49%, respectively. In addition, there was a 98% increase in intentional poisonings among 5 to 19 year-olds in New South Wales and Victoria between 2006 and 2016, with substantial overlap between the most commonly dispensed psychotropics and the drugs most commonly used in self-poisoning. These results do not support claims that increased antidepressant use reduces youth suicide risk. They are more consistent with the FDA warning and the hypothesis that antidepressant use increases the risk of suicide and self-harm by young people. Causal relationships cannot be established with certainty until there is a vast improvement in post-marketing surveillance. However, there is clear evidence that more young Australians are taking antidepressants, and more young Australians are killing themselves and self-harming, often by intentionally overdosing on the very substances that are supposed to help them.
Keywords: Australia; US Food and Drug Administration; adolescents; antidepressants; backlash; children; self-harm; suicide.
Copyright © 2020 Whitely, Raven and Jureidini.
Figures

References
-
- Food and Drug Administration Revisions to Product Labeling: Suicidality and Antidepressant Drugs. Rockville, MD: Food and Drug Administration; (2004/2007). Available at: http://www.fda.gov/downloads/drugs/drugsafety/informationbydrugclass/ucm... Accessed 16 March 2019.
-
- Food and Drug Administration Antidepressant Use in Children, Adolescents, and Adults. Rockville MD, USA: Food and Drug Administration; (2007). Available at https://wayback.archive-it.org/7993/20171101224428/https:/www.fda.gov/Dr... Accessed 15 April 2020.
Publication types
LinkOut - more resources
Full Text Sources

