miR-339 Promotes Hypoxia-Induced Neuronal Apoptosis and Impairs Cell Viability by Targeting FGF9/CACNG2 and Mediating MAPK Pathway in Ischemic Stroke
- PMID: 32587563
- PMCID: PMC7297914
- DOI: 10.3389/fneur.2020.00436
miR-339 Promotes Hypoxia-Induced Neuronal Apoptosis and Impairs Cell Viability by Targeting FGF9/CACNG2 and Mediating MAPK Pathway in Ischemic Stroke
Abstract
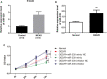
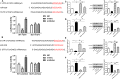
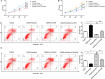
Ischemic stroke (IS) is a common cerebrovascular disease characterized by insufficient blood blow to the brain and the second leading cause of death as well as disability worldwide. Recent literatures have indicated that abnormal expression of miR-339 is closely related to IS. In this study, we attempted to assess the biological function of miR-339 and its underlying mechanism in IS. By accessing the GEO repository, the expression of miR-339, FGF9, and CACNG2 in middle cerebral artery occlusion (MCAO) and non-MCAO was evaluated. PC12 cells after oxygen-glucose deprivation/reoxygenation (OGD/R) treatment were prepared to mimic in vitro the IS model. The levels of miR-339, FGF9, CACNG2, and MAPK-related markers were quantitatively measured by qRT-PCR and Western blot. CCK-8 and flow cytometry analyses were performed to examine cell viability and apoptosis, respectively. IS-related potential pathways were identified using KEGG enrichment analysis and GO annotations. Bioinformatics analysis and dual-luciferase reporter assay were used to predict and verify the possible target of miR-339. Our results showed that miR-339 expression was significantly increased in MCAO and OGD/R-treated PC12 cells. Overexpression of miR-339 inhibited cell viability of PC12 cells subjected to OGD/R treatment. FGF9 and CACMG2 are direct targets of miR-339 and can reverse the aggressive effect of miR-339 on the proliferation and apoptosis of OGD/R-treated PC12 cells. Moreover, miR-339 mediated the activation of the MAPK pathway, which was inhibited by the FGF9/CACNG2 axis in PC12 cells treated by OGD/R stimulation. In summary, these findings suggested that miR-339 might act as a disruptive molecule to accelerate the IS progression via targeting the FGF9/CACNG2 axis and mediating the MAPK pathway.
Keywords: FGF9/CACNG2; MAPK pathway; apoptosis; ischemic stroke; miR-339; oxygen-glucose deprivation/reoxygenation (OGD/R).
Copyright © 2020 Gao, Ma and Zhang.
Figures



References
LinkOut - more resources
Full Text Sources

