Involvement of PaSNF1 in Fungal Development, Sterigmatocystin Biosynthesis, and Lignocellulosic Degradation in the Filamentous Fungus Podospora anserina
- PMID: 32587577
- PMCID: PMC7299030
- DOI: 10.3389/fmicb.2020.01038
Involvement of PaSNF1 in Fungal Development, Sterigmatocystin Biosynthesis, and Lignocellulosic Degradation in the Filamentous Fungus Podospora anserina
Abstract
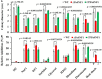
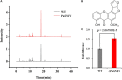
The sucrose non-fermenting 1/AMP-activated protein kinase (SNF1/AMPK) is a central regulator of carbon metabolism and energy production in the eukaryotes. In this study, the functions of the Podospora anserina SNF1 (PaSNF1) ortholog were investigated. The ΔPaSNF1 mutant displays a delayed development of mycelium and fruiting bodies and fails to form ascospores. The expression of the PaSNF1 gene in the strain providing female organs in a cross is sufficient to ensure fertility, indicating a maternal effect. Results of environmental stress showed that ΔPaSNF1 was hypersensitive to stress, such as osmotic pressure and heat shock, and resistant to fluconazole. Interestingly, the knockout of PaSNF1 significantly promoted sterigmatocystin (ST) synthesis but suppressed cellulase [filter paperase (FPA), endoglucanase (EG), and β-glucosidase (BG)] activity. Further, transcriptome analysis indicated that PaSNF1 made positive regulatory effects on the expression of genes encoding cellulolytic enzymes. These results suggested that PaSNF1 may function in balancing the operation of primary and secondary metabolism. This study suggested that SNF1 was a key regulator concerting vegetative growth, sexual development, and stress tolerance. Our study provided the first genetic evidence that SNF1 was involved in the ST biosynthesis and that it may also be a major actor of lignocellulose degradation in P. anserina.
Keywords: Podospora anserina; lignocellulose degradation; secondary metabolism; sexual development; stress tolerance; sucrose non-fermenting 1.
Copyright © 2020 Li, Yan, Lu, Qiu, Liang, Liu, Li, Mou and Xie.
Figures





Similar articles
-
Simultaneous Ablation of the Catalytic AMPK α-Subunit SNF1 and Mitochondrial Matrix Protease CLPP Results in Pronounced Lifespan Extension.Front Cell Dev Biol. 2021 Mar 4;9:616520. doi: 10.3389/fcell.2021.616520. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748105 Free PMC article.
-
Functional Characterization of the GATA-Type Transcription Factor PaNsdD in the Filamentous Fungus Podospora anserina and Its Interplay with the Sterigmatocystin Pathway.Appl Environ Microbiol. 2022 Mar 22;88(6):e0237821. doi: 10.1128/aem.02378-21. Epub 2022 Jan 26. Appl Environ Microbiol. 2022. PMID: 35080910 Free PMC article.
-
Functional characterization of the sterigmatocystin secondary metabolite gene cluster in the filamentous fungus Podospora anserina: involvement in oxidative stress response, sexual development, pigmentation and interspecific competitions.Environ Microbiol. 2019 Aug;21(8):3011-3026. doi: 10.1111/1462-2920.14698. Epub 2019 Jun 19. Environ Microbiol. 2019. PMID: 31136075
-
Plant biomass degrading ability of the coprophilic ascomycete fungus Podospora anserina.Biotechnol Adv. 2016 Sep-Oct;34(5):976-983. doi: 10.1016/j.biotechadv.2016.05.010. Epub 2016 Jun 1. Biotechnol Adv. 2016. PMID: 27263000 Review.
-
Mitochondrial metabolism and aging in the filamentous fungus Podospora anserina.Biochim Biophys Acta. 2006 May-Jun;1757(5-6):604-10. doi: 10.1016/j.bbabio.2006.03.005. Epub 2006 Mar 30. Biochim Biophys Acta. 2006. PMID: 16624249 Review.
Cited by
-
Current Advances in the Functional Genes of Edible and Medicinal Fungi: Research Techniques, Functional Analysis, and Prospects.J Fungi (Basel). 2024 Apr 25;10(5):311. doi: 10.3390/jof10050311. J Fungi (Basel). 2024. PMID: 38786666 Free PMC article. Review.
-
Simultaneous Ablation of the Catalytic AMPK α-Subunit SNF1 and Mitochondrial Matrix Protease CLPP Results in Pronounced Lifespan Extension.Front Cell Dev Biol. 2021 Mar 4;9:616520. doi: 10.3389/fcell.2021.616520. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748105 Free PMC article.
-
Genomic footprints related with adaptation and fumonisins production in Fusarium proliferatum.Front Microbiol. 2022 Sep 21;13:1004454. doi: 10.3389/fmicb.2022.1004454. eCollection 2022. Front Microbiol. 2022. PMID: 36212817 Free PMC article.
-
Sucrose non-fermenting protein kinase gene UvSnf1 is required for virulence in Ustilaginoidea virens.Virulence. 2023 Dec;14(1):2235460. doi: 10.1080/21505594.2023.2235460. Virulence. 2023. PMID: 37450576 Free PMC article.
-
Snf1 Kinase Differentially Regulates Botrytis cinerea Pathogenicity according to the Plant Host.Microorganisms. 2022 Feb 15;10(2):444. doi: 10.3390/microorganisms10020444. Microorganisms. 2022. PMID: 35208900 Free PMC article.
References
-
- Bills G. F., Gloer J. B. (2016). Biologically active secondary metabolites from the fungi. Microbiol. Spectr. 4:FUNK-0009-2016. - PubMed
LinkOut - more resources
Full Text Sources

