Human Adipose Derived Cells in Two- and Three-Dimensional Cultures: Functional Validation of an In Vitro Fat Construct
- PMID: 32587620
- PMCID: PMC7303735
- DOI: 10.1155/2020/4242130
Human Adipose Derived Cells in Two- and Three-Dimensional Cultures: Functional Validation of an In Vitro Fat Construct
Abstract
Obesity, defined as a body mass index of 30 kg/m2 or above, has increased considerably in incidence and frequency within the United States and globally. Associated comorbidities including cardiovascular disease, type 2 diabetes mellitus, metabolic syndrome, and nonalcoholic fatty liver disease have led to a focus on the mechanisms promoting the prevention and treatment of obesity. Commonly utilized in vitro models employ human or mouse preadipocyte cell lines in a 2-dimensional (2D) format. Due to the structural, biochemical, and biological limitations of these models, increased attention has been placed on "organ on a chip" technologies for a 3-dimensional (3D) culture. Herein, we describe a method employing cryopreserved primary human stromal vascular fraction (SVF) cells and a human blood product-derived biological scaffold to create a 3D adipose depot in vitro. The "fat-on-chip" 3D cultures have been validated relative to 2D cultures based on proliferation, flow cytometry, adipogenic differentiation, confocal microscopy/immunofluorescence, and functional assays (adipokine secretion, glucose uptake, and lipolysis). Thus, the in vitro culture system demonstrates the critical characteristics required for a humanized 3D white adipose tissue (WAT) model.
Copyright © 2020 Robert Bender et al.
Conflict of interest statement
The following authors state the following conflicts of interest: A. Alarcon, T. Frazier, J. M. Gimble, and X. Wu are all employees of LaCell LLC and Obatala Sciences Inc.; T. Frazier, J.M. Gimble, and X. Wu are cofounders and coowners of Obatala Sciences; J.M. Gimble and X. Wu are coowners and cofounders of LaCell. All remaining authors have no conflicts to declare.
Figures

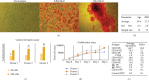
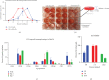
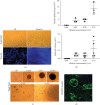
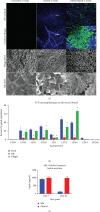


References
-
- Bourin P., Bunnell B. A., Casteilla L., et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/j.jcyt.2013.02.006. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

