Four and a half LIM domains protein 1 can be as a double-edged sword in cancer progression
- PMID: 32587768
- PMCID: PMC7309467
- DOI: 10.20892/j.issn.2095-3941.2019.0420
Four and a half LIM domains protein 1 can be as a double-edged sword in cancer progression
Abstract
Four and a half LIM domains protein 1 (FHL1), as the name suggests, contains four and a half LIM domains capable of interacting with various molecules, including structural proteins, kinases, and transcriptional machinery. FHL1 contains a zinc-finger domain and performs diverse roles in regulation of gene transcription, cytoarchitecture, cell proliferation, and signal transduction. Several studies have validated the importance of FHL1 in muscle development, myopathy, and cardiovascular diseases. Mutations in the FHL1 gene are associated with various myopathies. Recently, FHL1 was identified as a major host factor for chikungunya virus (CHIKV) infection in both humans and mice. Based on more recent findings over the last decade, FHL1 is proposed to play a dual role in cancer progression. On the one hand, FHL1 expression is suppressed in several cancer types, which correlates with increased metastatic disease and decreased survival. Moreover, FHL1 is reported to inhibit tumor cell growth and migration by associating with diverse signals, such as TGF-β and ER, and therefore considered a tumor suppressor. On the other hand, FHL1 can function as an oncogenic protein that promotes tumor progression upon phosphorylation, reflecting complex roles in cancer. This review primarily focuses on the dual role and underlying mechanisms of action of FHL1 in human cancer progression and its clinical relevance.
Keywords: Four and a half LIM protein 1 (FHL1); metastasis; tumor cell growth.
Copyright: © 2020, Cancer Biology & Medicine.
Figures
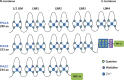
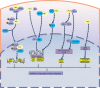
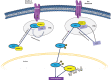
References
-
- Chan KK, Tsui SK, Lee SM, Luk SC, Liew CC, Fung KP, et al. Molecular cloning and characterization of FHL2, a novel LIM domain protein preferentially expressed in human heart. Gene. 1998;210:345–50. - PubMed
-
- Morgan MJ, Madgwick AJ. The fourth member of the FHL family of LIM proteins is expressed exclusively in the testis. Biochem Biophys Res Commun. 1999;255:251–5. - PubMed
-
- Morgan MJ, Madgwick AJ, Charleston B, Pell JM, Loughna PT. The developmental regulation of a novel muscle LIM-protein. Biochem Biophys Res Commun. 1995;212:840–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
