Heat shock protein 47 promotes tumor survival and therapy resistance by modulating AKT signaling via PHLPP1 in colorectal cancer
- PMID: 32587773
- PMCID: PMC7309463
- DOI: 10.20892/j.issn.2095-3941.2019.0261
Heat shock protein 47 promotes tumor survival and therapy resistance by modulating AKT signaling via PHLPP1 in colorectal cancer
Abstract
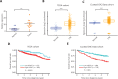
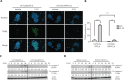
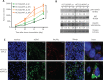
Objective: Heat shock protein 47 (HSP47) is a collagen-specific molecular chaperone that facilitates collagen maturation. Its role in cancer remains largely unknown. In this study, we investigated the roles of HSP47 in colorectal cancer (CRC) and therapy resistance. Methods: Expression of HSP47 in CRC tissues was examined (1) in paired human CRC/adjacent normal tissues, using real time quantitative reverse transcription polymerase chain reaction (qRT-PCR), The Cancer Genome Atlas (TCGA) database, and 22 independent microarray databases (curated CRC). In vitro studies on several CRC cell lines (HCT116, RKO and CCL228) with modulated HSP47 expression were conducted to assess cell viability and apoptosis (TUNEL assay and caspase-3/-7) during exposure to chemotherapy. AKT signaling and co-immunoprecipitation studies were performed to examine HSP47 and PHLPP1 interaction. In vivo studies using tumor xenografts were conducted to assess the effects of HSP47 modulation on tumor growth and therapy response. Results: HSP47 was upregulated in CRC and was associated with poor prognosis in individuals with CRC. In vitro, HSP47 overexpression supported the survival of CRC cells, whereas its knockdown sensitized cells to 5-fluorouracil (5-FU). HSP47 promoted survival by inhibiting apoptosis, enhancing AKT phosphorylation, and decreasing expression of the AKT-specific phosphatase PHLPP1 when cells were exposed to chemotherapy. These effects were partly results of the interaction between HSP47 and PHLPP1, which decreased PHLPP1 stability and led to more persistent AKT activity. In vivo, HSP47 supported tumor growth despite 5-FU treatment. Conclusions: HSP47 supports the growth of CRC tumors and suppresses the efficacy of chemotherapy via modulation of AKT signaling.
Keywords: AKT; HSP47; PHLPP1; colorectal cancer; resistanc.
Copyright: © 2020, Cancer Biology & Medicine.
Figures




Similar articles
-
HSP47 in human diseases: Navigating pathophysiology, diagnosis and therapy.Clin Transl Med. 2024 Aug;14(8):e1755. doi: 10.1002/ctm2.1755. Clin Transl Med. 2024. PMID: 39135385 Free PMC article. Review.
-
PHLPP is a negative regulator of RAF1, which reduces colorectal cancer cell motility and prevents tumor progression in mice.Gastroenterology. 2014 May;146(5):1301-12.e1-10. doi: 10.1053/j.gastro.2014.02.003. Epub 2014 Feb 11. Gastroenterology. 2014. PMID: 24530606 Free PMC article.
-
AHNAK2 confers 5-fluorouracil resistance in colorectal cancer via activation of the AKT/GSK-3β signaling axis.Clin Exp Med. 2025 May 18;25(1):168. doi: 10.1007/s10238-025-01682-3. Clin Exp Med. 2025. PMID: 40382757 Free PMC article.
-
Down-regulation of long non-coding RNA RP11-708H21.4 is associated with poor prognosis for colorectal cancer and promotes tumorigenesis through regulating AKT/mTOR pathway.Oncotarget. 2017 Apr 25;8(17):27929-27942. doi: 10.18632/oncotarget.15846. Oncotarget. 2017. PMID: 28427191 Free PMC article.
-
PHLiPPing the switch on Akt and protein kinase C signaling.Trends Endocrinol Metab. 2008 Aug;19(6):223-30. doi: 10.1016/j.tem.2008.04.001. Epub 2008 May 27. Trends Endocrinol Metab. 2008. PMID: 18511290 Free PMC article. Review.
Cited by
-
Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities.MedComm (2020). 2022 Aug 2;3(3):e161. doi: 10.1002/mco2.161. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35928554 Free PMC article. Review.
-
Adaptive response of resistant cancer cells to chemotherapy.Cancer Biol Med. 2020 Nov 15;17(4):842-863. doi: 10.20892/j.issn.2095-3941.2020.0005. Epub 2020 Dec 15. Cancer Biol Med. 2020. PMID: 33299639 Free PMC article. Review.
-
SERPINH1 promoted the proliferation and metastasis of colorectal cancer by activating PI3K/Akt/mTOR signaling pathway.World J Gastrointest Oncol. 2024 May 15;16(5):1890-1907. doi: 10.4251/wjgo.v16.i5.1890. World J Gastrointest Oncol. 2024. PMID: 38764814 Free PMC article.
-
HSP47 in human diseases: Navigating pathophysiology, diagnosis and therapy.Clin Transl Med. 2024 Aug;14(8):e1755. doi: 10.1002/ctm2.1755. Clin Transl Med. 2024. PMID: 39135385 Free PMC article. Review.
-
A positive feedback loop between SERPINH1 and MMP-9/TGF-β1 promotes lung adenocarcinoma progression.Cell Death Differ. 2025 Aug 16. doi: 10.1038/s41418-025-01558-9. Online ahead of print. Cell Death Differ. 2025. PMID: 40819105
References
-
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. - PubMed
-
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1-9. - PubMed
-
- Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, et al. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–9. - PubMed
-
- Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241–8. - PubMed
-
- Zhang J, Roberts TM, Shivdasani RA. Targeting PI3K signaling as a therapeutic approach for colorectal cancer. Gastroenterology. 2011;141:50–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
