DYRK1A suppression restrains Mcl-1 expression and sensitizes NSCLC cells to Bcl-2 inhibitors
- PMID: 32587776
- PMCID: PMC7309455
- DOI: 10.20892/j.issn.2095-3941.2019.0380
DYRK1A suppression restrains Mcl-1 expression and sensitizes NSCLC cells to Bcl-2 inhibitors
Abstract
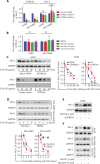
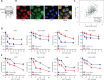
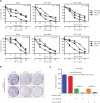
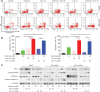
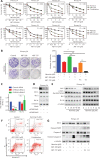
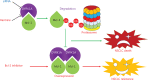
Objective: Mcl-1 overexpression confers acquired resistance to Bcl-2 inhibitors in non-small cell lung cancer (NSCLC), but no direct Mcl-1 inhibitor is currently available for clinical use. Thus, novel therapeutic strategies are urgently needed to target Mcl-1 and sensitize the anti-NSCLC activity of Bcl-2 inhibitors. Methods: Cell proliferation was measured using sulforhodamine B and colony formation assays, and apoptosis was detected with Annexin V-FITC staining. Gene expression was manipulated using siRNAs and plasmids. Real-time PCR and Western blot were used to measure mRNA and protein levels. Immunoprecipitation and immunofluorescence were used to analyze co-localization of dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A) and Mcl-1. Results: Suppression of DYRK1A resulted in reduced Mcl-1 expression in NSCLC cells, whereas overexpression of DYRK1A significantly increased Mcl-1 expression. Suppression of DYRK1A did not alter Mcl-1 mRNA levels, but did result in an accelerated degradation of Mcl-1 protein in NSCLC cells. Furthermore, DYRK1A mediated proteasome-dependent degradation of Mcl-1 in NSCLC cells, and DYRK1A co-localized with Mcl-1 in NSCLC cells and was co-expressed with Mcl-1 in tumor samples from lung cancer patients, suggesting that Mcl-1 may be a novel DYRK1A substrate. We showed that combined therapy with harmine and Bcl-2 antagonists significantly inhibited cell proliferation and induced apoptosis in NSCLC cell lines as well as primary NSCLC cells. Conclusions: Mcl-1 is a novel DYRK1A substrate, and the role of DYRK1A in promoting Mcl-1 stability makes it an attractive target for decreasing Bcl-2 inhibitor resistance.
Keywords: Bcl-2 inhibitor; DYRK1A; Mcl-1; NSCLC; combination.
Copyright: © 2020, Cancer Biology & Medicine.
Figures

References
-
- Lever JR, Fergason-Cantrell EA. Allosteric modulation of sigma receptors by BH3 mimetics ABT-737, ABT-263 (Navitoclax) and ABT-199 (Venetoclax). Pharmacol Res. 2019;142:87–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
