Clinical study of apatinib in the treatment of stage IV osteogenic sarcoma after failure of chemotherapy
- PMID: 32587785
- PMCID: PMC7309459
- DOI: 10.20892/j.issn.2095-3941.2019.0397
Clinical study of apatinib in the treatment of stage IV osteogenic sarcoma after failure of chemotherapy
Abstract
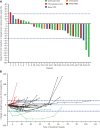
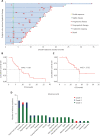
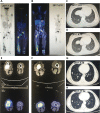
Objective: To analyze the efficacy and safety of apatinib in the treatment of stage IV osteogenic sarcoma after chemotherapy failure through a single-arm, prospective, and open clinical phase II study. Methods: Information on 34 patients with stage IV osteogenic sarcoma treated with apatinib after failure of chemotherapy in Tianjin Medical University Cancer Institute and Hospital between September 2015 and December 2019 was collected and analyzed. The participants included 23 males and 11 females, with an average age of 35.24 years (11-73 years). The objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), PFS rate (PFR), and overall survival (OS) were evaluated. The treatment-related adverse events (AEs) and safety of apatinib were also evaluated. Results: Of the 34 patients, 33 were able to be evaluated for efficacy. One patient received apatinib treatment for less than one cycle; therefore, only safety analysis was performed. The 12-week clinical evaluation showed that 2 patients had a partial response (PR), 24 patients had stable disease (SD), and 7 patients had progressive disease (PD). The ORR, DCR, and PFR at 12 weeks were 6.06% (2/33), 78.79% (26/33), and 82%, respectively. By the end of the follow-up, 6 patients had SD (18.18%, 6/33), 27 patients had PD (81.82%, 27/33), and 15 patients died because of disease progression (45.45%, 15/33). The ORR was 0 (0/33), the DCR was 18.18% (6/33), and the median PFS (mPFS) was 7.89 months (95% CI: 4.56-11.21). The median OS (mOS) was 17.61 months (95% CI: 10.85-24.37). The most common treatment-related AEs were hand-foot syndrome (35.29%, 12/34), proteinuria (32.35%, 11/34), and hypertension (32.35%, 11/34). Conclusions: Apatinib is effective and well tolerated in stage IV osteogenic sarcoma patients after chemotherapy failure.
Keywords: Apatinib; osteogenic sarcoma; progression-free survival; safety.
Copyright: © 2020, Cancer Biology & Medicine.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in china, 2015. CA Cancer J Clin. 2016;66:115–32. - PubMed
-
- Federman N, Bernthal N, Eilber FC, Tap WD. The multidisciplinary management of osteosarcoma. Curr Treat Options Oncol. 2009;10:82–93. - PubMed
-
- Ferrari S, Briccoli A, Mercuri M, Bertoni F, Picci P, Tienghi A, et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J Clin Oncol. 2003;21:710–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
