Development of a novel in vitro insulin resistance model in primary human tenocytes for diabetic tendinopathy research
- PMID: 32587790
- PMCID: PMC7304430
- DOI: 10.7717/peerj.8740
Development of a novel in vitro insulin resistance model in primary human tenocytes for diabetic tendinopathy research
Abstract
Background: Type 2 diabetes mellitus (T2DM) had been reported to be associated with tendinopathy. However, the underlying mechanisms of diabetic tendinopathy still remain largely to be discovered. The purpose of this study was to develop insulin resistance (IR) model on primary human tenocytes (hTeno) culture with tumour necrosis factor-alpha (TNF-α) treatment to study tenocytes homeostasis as an implication for diabetic tendinopathy.
Methods: hTenowere isolated from human hamstring tendon. Presence of insulin receptor beta (INSR-β) on normal tendon tissues and the hTeno monolayer culture were analyzed by immunofluorescence staining. The presence of Glucose Transporter Type 1 (GLUT1) and Glucose Transporter Type 4 (GLUT4) on the hTeno monolayer culture were also analyzed by immunofluorescence staining. Primary hTeno were treated with 0.008, 0.08, 0.8 and 8.0 µM of TNF-α, with and without insulin supplement. Outcome measures include 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose (2-NBDG) assay to determine the glucose uptake activity; colourimetric total collagen assay to quantify the total collagen expression levels; COL-I ELISA assay to measure the COL-I expression levels and real-time qPCR to analyze the mRNA gene expressions levels of Scleraxis (SCX), Mohawk (MKX), type I collagen (COL1A1), type III collagen (COL3A1), matrix metalloproteinases (MMP)-9 and MMP-13 in hTeno when treated with TNF-α. Apoptosis assay for hTeno induced with TNF-α was conducted using Annexin-V FITC flow cytometry analysis.
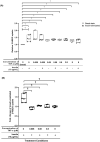
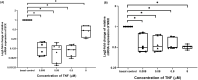
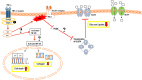
Results: Immunofluorescence imaging showed the presence of INSR-β on the hTeno in the human Achilles tendon tissues and in the hTeno in monolayer culture. GLUT1 and GLUT4 were both positively expressed in the hTeno. TNF-α significantly reduced the insulin-mediated 2-NBDG uptake in all the tested concentrations, especially at 0.008 µM. Total collagen expression levels and COL-I expression levels in hTeno were also significantly reduced in hTeno treated with 0.008 µM of TNF-α. The SCX, MKX and COL1A1 mRNA expression levels were significantly downregulated in all TNF-α treated hTeno, whereas the COL3A1, MMP-9 and MMP-13 were significantly upregulated in the TNF-α treated cells. TNF-α progressively increased the apoptotic cells at 48 and 72 h.
Conclusion: At 0.008 µM of TNF-α, an IR condition was induced in hTeno, supported with the significant reduction in glucose uptake, as well as significantly reduced total collagen, specifically COL-I expression levels, downregulation of candidate tenogenic markers genes (SCX and MKX), and upregulation of ECM catabolic genes (MMP-9 and MMP-13). Development of novel IR model in hTeno provides an insight on how tendon homeostasis could be affected and can be used as a tool for further discovering the effects on downstream molecular pathways, as the implication for diabetic tendinopathy.
Keywords: Cellular biology; Glucose uptake; Hyperglycemia; Insulin resistance; Obese; Orthopaedics; Tendon; Tenocyte; Tumor necrosis factor-alpha (TNF-α); Type II diabetes.
©2020 Tan et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures









References
-
- Ågren MS, Schnabel R, Christensen LH, Mirastschijski U. Tumor necrosis factor-α-accelerated degradation of type I collagen in human skin is associated with elevated matrix metalloproteinase (MMP)-1 and MMP-3 ex vivo. European Journal of Cell Biology. 2015;94:12–21. doi: 10.1016/j.ejcb.2014.10.001. - DOI - PMC - PubMed
-
- Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clinical Science. 1999;96:513–523. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

