Generation of Novel Plasmodium falciparum NF135 and NF54 Lines Expressing Fluorescent Reporter Proteins Under the Control of Strong and Constitutive Promoters
- PMID: 32587831
- PMCID: PMC7298075
- DOI: 10.3389/fcimb.2020.00270
Generation of Novel Plasmodium falciparum NF135 and NF54 Lines Expressing Fluorescent Reporter Proteins Under the Control of Strong and Constitutive Promoters
Abstract
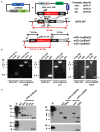
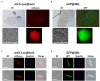
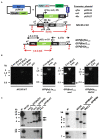
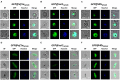
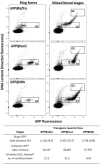
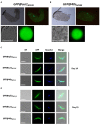
Transgenic reporter lines of malaria parasites that express fluorescent or luminescent proteins are valuable tools for drug and vaccine screening assays as well as to interrogate parasite gene function. Different Plasmodium falciparum (Pf ) reporter lines exist, however nearly all have been created in the African NF54/3D7 laboratory strain. Here we describe the generation of novel reporter lines, using CRISPR/Cas9 gene modification, both in the standard Pf NF54 background and in a recently described Cambodian P. falciparum NF135.C10 line. Sporozoites of this line show more effective hepatocyte invasion and enhanced liver merozoite development compared to Pf NF54. We first generated Pf NF54 reporter parasites to analyze two novel promoters for constitutive and high expression of mCherry-luciferase and GFP in blood and mosquito stages. The promoter sequences were selected based on available transcriptome data and are derived from two housekeeping genes, i.e., translation initiation factor SUI1, putative (sui1, PF3D7_1243600) and 40S ribosomal protein S30 (40s, PF3D7_0219200). We then generated and characterized reporter lines in the Pf NF135.C10 line which express GFP driven by the sui1 and 40s promoters as well as by the previously used ef1α promoter (GFP@ef1α, GFP@sui1, GFP@40s). The GFP@40s reporter line showed strongest GFP expression in liver stages as compared to the other two lines. The strength of reporter expression by the 40s promoter throughout the complete life cycle, including liver stages, makes transgenic lines expressing reporters by the 40s promoter valuable novel tools for analyses of P. falciparum.
Keywords: CRISPR/Cas9; NF135; Plasmodium falciparum; liver stage; malaria; reporter lines.
Copyright © 2020 Miyazaki, Yang, Geurten, Marin-Mogollon, Miyazaki, Imai, Kolli, Ramesar, Chevalley-Maurel, Salman, van Gemert, van Waardenburg, Franke-Fayard, Hill, Sauerwein, Janse and Khan.
Figures


Similar articles
-
Let it glow: genetically encoded fluorescent reporters in Plasmodium.Malar J. 2024 Apr 20;23(1):114. doi: 10.1186/s12936-024-04936-9. Malar J. 2024. PMID: 38643106 Free PMC article. Review.
-
A P. falciparum NF54 Reporter Line Expressing mCherry-Luciferase in Gametocytes, Sporozoites, and Liver-Stages.Front Cell Infect Microbiol. 2019 Apr 16;9:96. doi: 10.3389/fcimb.2019.00096. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31058097 Free PMC article.
-
Rapid Generation of Marker-Free P. falciparum Fluorescent Reporter Lines Using Modified CRISPR/Cas9 Constructs and Selection Protocol.PLoS One. 2016 Dec 20;11(12):e0168362. doi: 10.1371/journal.pone.0168362. eCollection 2016. PLoS One. 2016. PMID: 27997583 Free PMC article.
-
A transgenic Plasmodium falciparum NF54 strain that expresses GFP-luciferase throughout the parasite life cycle.Mol Biochem Parasitol. 2012 Dec;186(2):143-7. doi: 10.1016/j.molbiopara.2012.10.004. Epub 2012 Oct 27. Mol Biochem Parasitol. 2012. PMID: 23107927
-
Reporter parasite lines: valuable tools for the study of Plasmodium biology.Trends Parasitol. 2024 Nov;40(11):1000-1015. doi: 10.1016/j.pt.2024.09.003. Epub 2024 Oct 9. Trends Parasitol. 2024. PMID: 39389901 Review.
Cited by
-
Creation and preclinical evaluation of genetically attenuated malaria parasites arresting growth late in the liver.NPJ Vaccines. 2022 Nov 4;7(1):139. doi: 10.1038/s41541-022-00558-x. NPJ Vaccines. 2022. PMID: 36333336 Free PMC article.
-
Generation of a Genetically Modified Chimeric Plasmodium falciparum Parasite Expressing Plasmodium vivax Circumsporozoite Protein for Malaria Vaccine Development.Front Cell Infect Microbiol. 2020 Dec 17;10:591046. doi: 10.3389/fcimb.2020.591046. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33392104 Free PMC article.
-
Let it glow: genetically encoded fluorescent reporters in Plasmodium.Malar J. 2024 Apr 20;23(1):114. doi: 10.1186/s12936-024-04936-9. Malar J. 2024. PMID: 38643106 Free PMC article. Review.
-
A versatile Plasmodium falciparum reporter line expressing NanoLuc enables highly sensitive multi-stage drug assays.Commun Biol. 2023 Jul 12;6(1):713. doi: 10.1038/s42003-023-05078-5. Commun Biol. 2023. PMID: 37438491 Free PMC article.
-
PfSPZ Vaccine induces focused humoral immune response in HIV positive and negative Tanzanian adults.EBioMedicine. 2024 Oct;108:105364. doi: 10.1016/j.ebiom.2024.105364. Epub 2024 Sep 30. EBioMedicine. 2024. PMID: 39353279 Free PMC article.
References
-
- Adjalley S. H., Johnston G. L., Li T., Eastman R. T., Ekland E. H., Eappen A. G., et al. . (2011). Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl. Acad. Sci. U.S.A. 108, E1214–E1223. 10.1073/pnas.1112037108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

