Magnesium Alloys With Tunable Interfaces as Bone Implant Materials
- PMID: 32587850
- PMCID: PMC7297987
- DOI: 10.3389/fbioe.2020.00564
Magnesium Alloys With Tunable Interfaces as Bone Implant Materials
Abstract
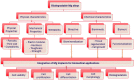
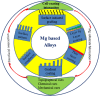
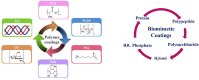
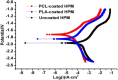
Magnesium (Mg) based biodegradable materials are a new generation orthopedic implant materials that are intended to possess same mechanical properties as that of bone. Mg alloys are considered as promising substitutes to permanent implants due to their biodegradability in the physiological environment. However, rapid corrosion rate is one of the major constraints of using Mg alloys in clinical applications in spite of their excellent biocompatibility. Approaches to overcome the limitations include the selection of adequate alloying elements, proper surface treatment, surface modification with coating to control the degradation rate. This review focuses on current advances on surface engineering of Mg based biomaterials for biomedical applications. The review begins with a description of corrosion mechanism of Mg alloy, the requirement for appropriate surface functionalization/coatings, their structure-property-performance relationship, and suitability for biomedical applications. The control of physico-chemical properties such as wettability, surface morphology, surface chemistry, and surface functional groups of the coating tailored by various approaches forms the pivotal part of the review. Chemical surface treatment offers initial protection from corrosion and inorganic coating like hydroxyapatite (HA) improves the biocompatibility of the substrate. Considering the demand of ideal implant materials, multilayer hybrid coatings on Mg alloy in combination with chemical pretreatment or inorganic HA coating, and protein-based polymer coating could be a promising technique to improve corrosion resistance and promote biocompatibility of Mg-based alloys.
Keywords: biomedical application; corrosion; interfacial engineering; magnesium alloy; surface coating.
Copyright © 2020 Rahman, Dutta and Roy Choudhury.
Figures









References
-
- Abdal-hay A., Barakat N. A., Lim J. K. (2013). Hydroxyapatite-doped poly (lactic acid) porous film coating for enhanced bioactivity and corrosion behavior of AZ31 Mg alloy for orthopedic applications. Ceram. Int. 39, 183–195. 10.1016/j.ceramint.2012.06.008 - DOI
-
- Abedalwafa M., Wang F., Wang L., Li C. (2013). Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: a review. Rev. Adv. Mater. Sci. 34, 123–140.
-
- Alsaheb R. A., Aladdin A., Othman N. Z., Malek R. A., Leng O. M., Aziz R., et al. (2015). Recent applications of polylactic acid in pharmaceutical and medical industries. J. Chem. Pharm. Res. 7, 51–63.
-
- Amaravathy P., Sathyanarayanan S., Sowndarya S., Rajendran N. (2014). Bioactive HA/TiO2 coating on magnesium alloy for biomedical applications. Ceram. Int. 40, 6617–6630. 10.1016/j.ceramint.2013.11.119 - DOI
Publication types
LinkOut - more resources
Full Text Sources

