Alcalase Microarray Base on Metal Ion Modified Hollow Mesoporous Silica Spheres as a Sustainable and Efficient Catalysis Platform for Proteolysis
- PMID: 32587851
- PMCID: PMC7297948
- DOI: 10.3389/fbioe.2020.00565
Alcalase Microarray Base on Metal Ion Modified Hollow Mesoporous Silica Spheres as a Sustainable and Efficient Catalysis Platform for Proteolysis
Abstract
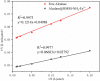
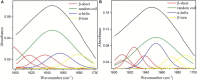
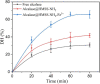
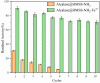
The industrial exploitation of protease is limited owing to its sensitivity to environmental factors and autolysis during biocatalytic processes. In the present study, the alcalase microarray (Bacillus licheniformis, alcalase@HMSS-NH2-Metal) based on different metal ions modified hollow mesoporous silica spheres (HMSS-NH2-Metal) was successfully developed via a facile approach. Among the alcalase@HMSS-NH2-Metal (Ca2+, Zn2+, Fe3+, Cu2+), the alcalase@HMSS-NH2-Fe3+ revealed the best immobilization efficiency and enzymatic properties. This tailor-made nanocomposite immobilized alcalase on a surface-bound network of amino-metal complex bearing protein-modifiable sites via metal-protein affinity. The coordination interaction between metal ion and alcalase advantageously changed the secondary structure of enzyme, thus significantly enhanced the bioactivities and thermostability of alcalase. The as-prepared alcalase@HMSS-NH2-Fe3+ exhibited excellent loading capacity (227.8 ± 23.7 mg/g) and proteolytic activity. Compared to free form, the amidase activity of alcalase microarray increased by 5.3-fold, the apparent kinetic constant Vmax/Km of alcalase@HMSS-NH2-Fe3+ (15.6 min-1) was 1.9-fold higher than that of free alcalase, and the biocatalysis efficiency increased by 2.1-fold for bovine serum albumin (BSA) digestion. Moreover, this particular immobilization strategy efficiently reduced the bioactivities losses of alcalase caused by enzyme leaking and autolysis during the catalytic process. The alcalase microarray still retained 70.7 ± 3.7% of the initial activity after 10 cycles of successive reuse. Overall, this study established a promising strategy to overcome disadvantages posed by free alcalase, which provided new expectations for the application of alcalase in sustainable and efficient proteolysis.
Keywords: alcalase immobilization; alcalase microarray; hollow mesoporous silica spheres (HMSS); metal ion modified nanocomposite; metal-protein affinity; proteolysis.
Copyright © 2020 Zeng, Li, Sun and Zheng.
Figures


Similar articles
-
Chitosan-capped enzyme-responsive hollow mesoporous silica nanoplatforms for colon-specific drug delivery.Nanoscale Res Lett. 2020 Jun 1;15(1):123. doi: 10.1186/s11671-020-03351-8. Nanoscale Res Lett. 2020. PMID: 32488526 Free PMC article.
-
Design of amino-functionalized hollow mesoporous silica cube for enzyme immobilization and its application in synthesis of phosphatidylserine.Colloids Surf B Biointerfaces. 2021 Jun;202:111668. doi: 10.1016/j.colsurfb.2021.111668. Epub 2021 Mar 1. Colloids Surf B Biointerfaces. 2021. PMID: 33740632
-
Yolk-shell Fe(0)@SiO2 nanoparticles as nanoreactors for fenton-like catalytic reaction.ACS Appl Mater Interfaces. 2014 Aug 13;6(15):13167-73. doi: 10.1021/am503063m. Epub 2014 Aug 1. ACS Appl Mater Interfaces. 2014. PMID: 25050829
-
Immobilization of Alcalase on Silica Supports Modified with Carbosilane and PAMAM Dendrimers.Int J Mol Sci. 2022 Dec 17;23(24):16102. doi: 10.3390/ijms232416102. Int J Mol Sci. 2022. PMID: 36555742 Free PMC article.
-
Eye-Readable Detection and Oxidation of CO with a Platinum-Based Catalyst and a Binuclear Rhodium Complex.Angew Chem Int Ed Engl. 2019 Aug 26;58(35):12258-12263. doi: 10.1002/anie.201905567. Epub 2019 Jul 22. Angew Chem Int Ed Engl. 2019. PMID: 31197913 Review.
Cited by
-
Partial enrichment of phospholipids by enzymatic hydrolysis and membrane filtration of whey protein phospholipid concentrate.JDS Commun. 2023 Feb 24;4(3):175-180. doi: 10.3168/jdsc.2022-0322. eCollection 2023 May. JDS Commun. 2023. PMID: 37360124 Free PMC article.
References
-
- Al-Oweini R., El-Rassy H. (2009). Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R″Si(OR′)3 precursors. J. Mol. Struct. 919, 140–145. 10.1016/j.molstruc.2008.08.025 - DOI
-
- Antecka K., Zdarta J., Siwinska-Stefanska K., Sztuk G., Jankowska E., Oleskowicz-Popiel P., et al. (2018). Synergistic degradation of dye wastewaters using binary or ternary oxide systems with immobilized laccase. Catalysts 8:402 10.3390/catal8090402 - DOI
-
- Bayramoglu G., Ozalp V. C., Arica M. Y. (2013). Magnetic polymeric beads functionalized with different mixed-mode ligands for reversible immobilization of trypsin. Ind. Eng. Chem. Res. 53, 132–140. 10.1021/ie402656p - DOI
-
- Bernal C., Poveda-Jaramillo J. C., Mesa M. (2018a). Raising the enzymatic performance of lipase and protease in the synthesis of sugar fatty acid esters, by combined ionic exchange -hydrophobic immobilization process on aminopropyl silica support. Chem. Eng. J. 334, 760–767. 10.1016/j.cej.2017.10.082 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

