The Parasitic Intracellular Lifestyle of Trypanosomatids: Parasitophorous Vacuole Development and Survival
- PMID: 32587854
- PMCID: PMC7297907
- DOI: 10.3389/fcell.2020.00396
The Parasitic Intracellular Lifestyle of Trypanosomatids: Parasitophorous Vacuole Development and Survival
Abstract
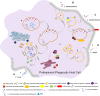
The trypanosomatid (protozoan) parasites Trypanosoma cruzi and Leishmania spp. are causative agents of Chagas disease and Leishmaniasis, respectively. They display high morphological plasticity, are capable of developing in both invertebrate and vertebrate hosts, and are the only trypanosomatids that can survive and multiply inside mammalian host cells. During internalization by host cells, these parasites are lodged in "parasitophorous vacuoles" (PVs) comprised of host cell endolysosomal system components. PVs effectively shelter parasites within the host cell. PV development and maturation (acidification, acquisition of membrane markers, and/or volumetric expansion) precede parasite escape from the vacuole and ultimately from the host cell, which are key determinants of infective burden and persistence. PV biogenesis varies, depending on trypanosomatid species, in terms of morphology (e.g., size), biochemical composition, and parasite-mediated processes that coopt host cell machinery. PVs play essential roles in the intracellular development (i.e., morphological differentiation and/or multiplication) of T. cruzi and Leishmania spp. They are of great research interest as potential gateways for drug delivery systems and other therapeutic strategies for suppression of parasite multiplication and control of the large spectrum of diseases caused by these trypanosomatids. This mini-review focuses on mechanisms of PV biogenesis, and processes whereby PVs of T. cruzi and Leishmania spp. promote parasite persistence within and dissemination among mammalian host cells.
Keywords: Leishmania; Trypanosoma cruzi; intracellular pathogen; parasitophorous vacuole; vacuole.
Copyright © 2020 Batista, Nájera, Meneghelli and Bahia.
Figures

Similar articles
-
Trypanosoma cruzi Differentiates and Multiplies within Chimeric Parasitophorous Vacuoles in Macrophages Coinfected with Leishmania amazonensis.Infect Immun. 2016 Apr 22;84(5):1603-1614. doi: 10.1128/IAI.01470-15. Print 2016 May. Infect Immun. 2016. PMID: 26975994 Free PMC article.
-
Structures containing galectin-3 are recruited to the parasitophorous vacuole containing Trypanosoma cruzi in mouse peritoneal macrophages.Parasitol Res. 2014 Jun;113(6):2323-33. doi: 10.1007/s00436-014-3887-8. Epub 2014 Apr 24. Parasitol Res. 2014. PMID: 24760627
-
Trypanosoma cruzi: Entry into Mammalian Host Cells and Parasitophorous Vacuole Formation.Front Immunol. 2013 Aug 1;4:186. doi: 10.3389/fimmu.2013.00186. eCollection 2013. Front Immunol. 2013. PMID: 23914186 Free PMC article.
-
[Formation and diversity of parasitophorous vacuoles in parasitic protozoa. The Coccidia (Sporozoa, Apicomplexa)].Tsitologiia. 2003;45(4):339-56. Tsitologiia. 2003. PMID: 14520865 Review. Russian.
-
Role of Virulence Factors of Trypanosomatids in the Insect Vector and Putative Genetic Events Involved in Surface Protein Diversity.Front Cell Infect Microbiol. 2022 Apr 28;12:807172. doi: 10.3389/fcimb.2022.807172. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35573777 Free PMC article. Review.
Cited by
-
Cell-Penetrating Antimicrobial Peptides with Anti-Infective Activity against Intracellular Pathogens.Antibiotics (Basel). 2022 Dec 8;11(12):1772. doi: 10.3390/antibiotics11121772. Antibiotics (Basel). 2022. PMID: 36551429 Free PMC article. Review.
-
The Glycan Structure of T. cruzi mucins Depends on the Host. Insights on the Chameleonic Galactose.Molecules. 2020 Aug 27;25(17):3913. doi: 10.3390/molecules25173913. Molecules. 2020. PMID: 32867240 Free PMC article. Review.
-
Host Organelle Interactions Facilitate Cholesterol Acquisition by Trypanosoma cruzi Amastigotes.J Eukaryot Microbiol. 2025 Jul-Aug;72(4):e70027. doi: 10.1111/jeu.70027. J Eukaryot Microbiol. 2025. PMID: 40685832 Free PMC article.
-
Alkyl-Resorcinol Derivatives as Inhibitors of GDP-Mannose Pyrophosphorylase with Antileishmanial Activities.Molecules. 2021 Mar 11;26(6):1551. doi: 10.3390/molecules26061551. Molecules. 2021. PMID: 33799883 Free PMC article.
-
Theileria's Strategies and Effector Mechanisms for Host Cell Transformation: From Invasion to Immortalization.Front Cell Dev Biol. 2021 Apr 20;9:662805. doi: 10.3389/fcell.2021.662805. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33959614 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

