Efficacy of 1-α-Hydroxycholecalciferol Supplementation in Young Broiler Feed Suggests Reducing Calcium Levels at Grower Phase
- PMID: 32587863
- PMCID: PMC7299047
- DOI: 10.3389/fvets.2020.00245
Efficacy of 1-α-Hydroxycholecalciferol Supplementation in Young Broiler Feed Suggests Reducing Calcium Levels at Grower Phase
Abstract
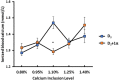
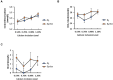
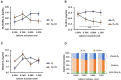
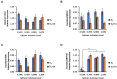
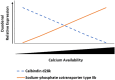
Increasing biopotency of cholecalciferol (D3) from vitamin sources is essential in the poultry industry to meet nutritional demands and counter stressors. D3 exhibits hormonal traits and is responsible for calcium (Ca) absorption. 1-α-Hydroxycholecalciferol (1α) is a synthetic form of D3 that has equal efficacy and is cheaper to synthesize than 1,25-dihydroxycholecalciferol (active form of D3), on broilers. However, 1α bypasses a critical regulatory point, the kidney, and may consequently lead to toxicity levels of Ca via Ca absorption. This study examined 1α supplementation in broiler diets with different Ca inclusion levels to determine if 1α at higher Ca levels caused Ca toxicity at starter and grower phases with Ross 708 male broiler chicks. In Experiment 1 (1-15 days of age), chicks were assigned to one of 10 treatment starter diets with five levels of Ca inclusion (0.80, 0.95, 1.10, 1.25, and 1.40%) with or without 1α supplementation (5 μg 1α/kg in feed) and eight replicate cages per treatment. In Experiment 2, chicks were fed common starter diet until 16 days of age, and then they were assigned to one of eight treatment diets with four levels of Ca inclusion (0.54, 0.76, 0.98, or 1.20%) with or without 1α supplementation (5 μg 1α/kg in feed). At the end of both experiments, blood was collected from broilers to determine blood chemistry, including concentrations of vitamin D metabolites. Intestinal tissues were also collected to examine gene expression. In Experiment 1, broilers not fed 1α exhibited a quadratic effect in ionized blood Ca (iCa) as dietary Ca inclusion levels increased; 1α-fed broilers displayed an increase in iCa as Ca inclusion levels increased (p = 0.0002). For Experiment 2, 1α-fed broilers displayed a decrease in 25-hydroxycholecalciferol plasma concentration as dietary Ca inclusion levels increased (p = 0.035); also, increasing Ca inclusion in diets increased expression of duodenal sodium phosphate cotransporter type II b (NPTIIb, p = 0.03). Our findings imply that inclusion of 1α was beneficial because 1α enhanced Ca absorption during the starter phase; however, to avoid potential Ca toxicity or antagonism while using 1α during the grower phase, there should be consideration with reducing dietary Ca levels.
Keywords: 1-α-hydroxycholecalciferol; 25-hydroxycholecalciferol; blood chemistry; broiler; calbindin d28k; calcium; sodium phosphate cotransporter type IIb; vitamin D3.
Copyright © 2020 Warren, Vu, Toomer, Fernandez and Livingston.
Figures

References
-
- Waldenstedt L. Nutritional factors of importance for optimal leg health in broilers: a review. Anim Feed Sci Technol. (2006) 126:291–307. 10.1016/j.anifeedsci.2005.08.008 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

