Sorting Out the Role of the Sortilin-Related Receptor 1 in Alzheimer's Disease
- PMID: 32587946
- PMCID: PMC7306921
- DOI: 10.3233/ADR-200177
Sorting Out the Role of the Sortilin-Related Receptor 1 in Alzheimer's Disease
Abstract
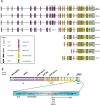
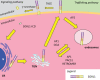
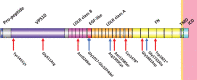
Sortilin-related receptor 1 (SORL1) encodes a large, multi-domain containing, membrane-bound receptor involved in endosomal sorting of proteins between the trans-Golgi network, endosomes and the plasma membrane. It is genetically associated with Alzheimer's disease (AD), the most common form of dementia. SORL1 is a unique gene in AD, as it appears to show strong associations with the common, late-onset, sporadic form of AD and the rare, early-onset familial form of AD. Here, we review the genetics of SORL1 in AD and discuss potential roles it could play in AD pathogenesis.
Keywords: Alzheimer’s disease; amyloid; amyloid-beta protein precursor; endocytosis; endosomes; protein transport.
© 2020 – IOS Press and the authors. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures


Similar articles
-
Brain Transcriptome Analysis of a Protein-Truncating Mutation in Sortilin-Related Receptor 1 Associated With Early-Onset Familial Alzheimer's Disease Indicates Early Effects on Mitochondrial and Ribosome Function.J Alzheimers Dis. 2021;79(3):1105-1119. doi: 10.3233/JAD-201383. J Alzheimers Dis. 2021. PMID: 33386808
-
The Role of SORL1 in Alzheimer's Disease.Mol Neurobiol. 2015;51(3):909-18. doi: 10.1007/s12035-014-8742-5. Epub 2014 May 16. Mol Neurobiol. 2015. PMID: 24833601 Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Sortilin-Related Receptor Expression in Human Neural Stem Cells Derived from Alzheimer's Disease Patients Carrying the APOE Epsilon 4 Allele.Neural Plast. 2017;2017:1892612. doi: 10.1155/2017/1892612. Epub 2017 May 28. Neural Plast. 2017. PMID: 28634550 Free PMC article.
-
A familial missense variant in the Alzheimer's Disease gene SORL1 impairs its maturation and endosomal sorting.bioRxiv [Preprint]. 2023 Nov 13:2023.07.01.547348. doi: 10.1101/2023.07.01.547348. bioRxiv. 2023. Update in: Acta Neuropathol. 2024 Jan 20;147(1):20. doi: 10.1007/s00401-023-02670-1. PMID: 37461597 Free PMC article. Updated. Preprint.
Cited by
-
Brain transcriptomes of zebrafish and mouse Alzheimer's disease knock-in models imply early disrupted energy metabolism.Dis Model Mech. 2022 Jan 1;15(1):dmm049187. doi: 10.1242/dmm.049187. Epub 2022 Jan 26. Dis Model Mech. 2022. PMID: 34842276 Free PMC article.
-
Systematic Analysis of Biological Processes Reveals Gene Co-expression Modules Driving Pathway Dysregulation in Alzheimer's Disease.Aging Dis. 2024 Jun 12;16(3):1598-1625. doi: 10.14336/AD.2024.0429. Aging Dis. 2024. PMID: 38913039 Free PMC article.
-
Endophenotypic effects of the SORL1 variant rs2298813 on regional brain volume in patients with late-onset Alzheimer's disease.Front Aging Neurosci. 2022 Aug 5;14:885090. doi: 10.3389/fnagi.2022.885090. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35992588 Free PMC article.
-
Genetic Markers of Postmortem Brain Iron.J Neurochem. 2025 Feb;169(2):e16309. doi: 10.1111/jnc.16309. J Neurochem. 2025. PMID: 39918201 Free PMC article.
-
Heterozygous and Homozygous Variants in SORL1 Gene in Alzheimer's Disease Patients: Clinical, Neuroimaging and Neuropathological Findings.Int J Mol Sci. 2022 Apr 11;23(8):4230. doi: 10.3390/ijms23084230. Int J Mol Sci. 2022. PMID: 35457051 Free PMC article.
References
-
- Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368, 387–403. - PubMed
-
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primers 1, 15056. - PubMed
-
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L (1992) A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N–terminus of β–amyloid. Nat Genet 1, 345–347. - PubMed
-
- Kumar-Singh S, De Jonghe C, Cruts M, Kleinert R, Wang R, Mercken M, De Strooper B, Vanderstichele H, Lofgren A, Vanderhoeven I, Backhovens H, Vanmechelen E, Kroisel PM, Van Broeckhoven C (2000) Nonfibrillar diffuse amyloid deposition due to a gamma(42)-secretase site mutation points to an essential role for N-truncated A beta(42) in Alzheimer’s disease. Hum Mol Genet 9, 2589–2598. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

