This is a preprint.
Detection of antibodies to the SARS-CoV-2 spike glycoprotein in both serum and saliva enhances detection of infection
- PMID: 32588002
- PMCID: PMC7310662
- DOI: 10.1101/2020.06.16.20133025
Detection of antibodies to the SARS-CoV-2 spike glycoprotein in both serum and saliva enhances detection of infection
Update in
-
Development of a high-sensitivity ELISA detecting IgG, IgA and IgM antibodies to the SARS-CoV-2 spike glycoprotein in serum and saliva.Immunology. 2021 Sep;164(1):135-147. doi: 10.1111/imm.13349. Epub 2021 May 24. Immunology. 2021. PMID: 33932228 Free PMC article.
Abstract
Background: Detecting antibody responses during and after SARS-CoV-2 infection is essential in determining the seroepidemiology of the virus and the potential role of antibody in disease. Scalable, sensitive and specific serological assays are essential to this process. The detection of antibody in hospitalized patients with severe disease has proven straightforward; detecting responses in subjects with mild disease and asymptomatic infections has proven less reliable. We hypothesized that the suboptimal sensitivity of antibody assays and the compartmentalization of the antibody response may contribute to this effect.
Methods: We systemically developed an ELISA assay, optimising different antigens and amplification steps, in serum and saliva from symptomatic and asymptomatic SARS-CoV-2-infected subjects.
Results: Using trimeric spike glycoprotein, rather than nucleocapsid enabled detection of responses in individuals with low antibody responses. IgG1 and IgG3 predominate to both antigens, but more anti-spike IgG1 than IgG3 was detectable. All antigens were effective for detecting responses in hospitalized patients. Anti-spike, but not nucleocapsid, IgG, IgA and IgM antibody responses were readily detectable in saliva from non-hospitalized symptomatic and asymptomatic individuals. Antibody responses in saliva and serum were largely independent of each other and symptom reporting.
Conclusions: Detecting antibody responses in both saliva and serum is optimal for determining virus exposure and understanding immune responses after SARS-CoV-2 infection.
Funding: This work was funded by the University of Birmingham, the National Institute for Health Research (UK), the NIH National Institute for Allergy and Infectious Diseases, the Bill and Melinda Gates Foundation and the University of Southampton.
Conflict of interest statement
Conflict of interest statement
AC and SH are employed by the Binding Site Group Ltd. AH has a commercial relationship with Binding Site Group Ltd. MTD and MG have a commercial relationship with Abingdon Health. The rest of the authors declared no conflict of interest.
Figures
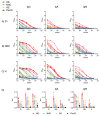
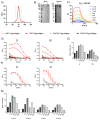
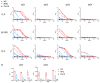
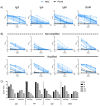


References
-
- WHO. Coronavirus disease (COVID-19) Situation Report - 141. 09/06/2020 2020;
-
- Shields AM, et al. SARS-CoV-2 seroconversion in health care workers. medRxiv. 2020:2020.05.18.20105197. doi: 10.1101/2020.05.18.20105197 - DOI
-
- Woo PC, et al. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polypeptide. J Clin Microbiol. December 2004;42(12):5885–8. doi: 10.1128/JCM.42.12.5885-5888.2004 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
