Ketogenic diets for drug-resistant epilepsy
- PMID: 32588435
- PMCID: PMC7387249
- DOI: 10.1002/14651858.CD001903.pub5
Ketogenic diets for drug-resistant epilepsy
Abstract
Background: Ketogenic diets (KDs) are high in fat and low in carbohydrates and have been suggested to reduce seizure frequency in people with epilepsy. Such diets may be beneficial for children with drug-resistant epilepsy. This is an update of a review first published in 2003, and last updated in 2018.
Objectives: To assess the effects of ketogenic diets for people with drug-resistant epilepsy.
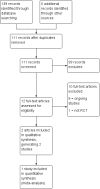
Search methods: For this update, we searched the Cochrane Register of Studies (CRS Web) and MEDLINE (Ovid, 1946 to 26 April 2019) on 29 April 2019. The Cochrane Register of Studies includes the Cochrane Epilepsy Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), and randomised controlled trials (RCTs) from Embase, ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We imposed no language restrictions. We checked the reference lists of retrieved studies for additional relevant studies.
Selection criteria: RCTs or quasi-RCTs of KDs for people of any age with drug-resistant epilepsy.
Data collection and analysis: Two review authors independently applied predefined criteria to extract data and evaluated study quality. We assessed the outcomes: seizure freedom, seizure reduction (50% or greater reduction in seizure frequency), adverse effects, cognition and behaviour, quality of life, and attrition rate. We incorporated a meta-analysis. We utilised an intention-to-treat (ITT) population for all primary analyses. We presented the results as risk ratios (RRs) with 95% confidence intervals (CIs).
Main results: We identified 13 studies with 932 participants; 711 children (4 months to 18 years) and 221 adults (16 years and over). We assessed all 13 studies to be at high risk of performance and detection bias, due to lack of blinding. Assessments varied from low to high risk of bias for all other domains. We rated the evidence for all outcomes as low to very low certainty. Ketogenic diets versus usual care for children Seizure freedom (RR 3.16, 95% CI 1.20 to 8.35; P = 0.02; 4 studies, 385 participants; very low-certainty evidence) and seizure reduction (RR 5.80, 95% CI 3.48 to 9.65; P < 0.001; 4 studies, 385 participants; low-certainty evidence) favoured KDs (including: classic KD, medium-chain triglyceride (MCT) KD combined, MCT KD only, simplified modified Atkins diet (MAD) compared to usual care for children. We are not confident that these estimated effects are accurate. The most commonly reported adverse effects were vomiting, constipation and diarrhoea for both the intervention and usual care group, but the true effect could be substantially different (low-certainty evidence). Ketogenic diet versus usual care for adults In adults, no participants experienced seizure freedom. Seizure reduction favoured KDs (MAD only) over usual care but, again, we are not confident that the effect estimated is accurate (RR 5.03, 95% CI 0.26 to 97.68; P = 0.29; 2 studies, 141 participants; very low-certainty evidence). Adults receiving MAD most commonly reported vomiting, constipation and diarrhoea (very low-certainty evidence). One study reported a reduction in body mass index (BMI) plus increased cholesterol in the MAD group. The other reported weight loss. The true effect could be substantially different to that reported. Ketogenic diet versus ketogenic diet for children Up to 55% of children achieved seizure freedom with a classical 4:1 KD after three months whilst up to 85% of children achieved seizure reduction (very low-certainty evidence). One trial reported a greater incidence of seizure reduction with gradual-onset KD, as opposed to fasting-onset KD. Up to 25% of children were seizure free with MAD and up to 60% achieved seizure reduction. Up to 25% of children became seizure free with MAD and up to 60% experienced seizure reduction. One study used a simplified MAD (sMAD) and reported that 15% of children gained seizure freedom rates and 56% achieved seizure reduction. We judged all the evidence described as very low certainty, thus we are very unsure whether the results are accurate. The most commonly reported adverse effects were vomiting, constipation and diarrhoea (5 studies, very low-certainty evidence). Two studies reported weight loss. One stated that weight loss and gastrointestinal disturbances were more frequent, with 4:1 versus 3:1 KD, whilst one reported no difference in weight loss with 20 mg/d versus 10 mg/d carbohydrates. In one study, there was a higher incidence of hypercalcuria amongst children receiving classic KD compared to MAD. All effects described are unlikely to be accurate. Ketogenic diet versus ketogenic diet for adults One study randomised 80 adults (aged 18 years and over) to either MAD plus KetoCal during the first month with MAD alone for the second month, or MAD alone for the first month followed by MAD plus KetoCal for the second month. No adults achieved seizure freedom. More adults achieved seizure reduction at one month with MAD alone (42.5%) compared to MAD plus KetoCal (32.5%), however, by three months only 10% of adults in both groups maintained seizure reduction. The evidence for both outcomes was of very low certainty; we are very uncertain whether the effects are accurate. Constipation was more frequently reported in the MAD plus KetoCal group (17.5%) compared to the MAD only group (5%) (1 study, very low-certainty evidence). Diarrhoea and increase/change in seizure pattern/semiology were also commonly reported (17.5% to 20% of participants). The true effects of the diets could be substantially different to that reported.
Authors' conclusions: The evidence suggests that KDs could demonstrate effectiveness in children with drug-resistant epilepsy, however, the evidence for the use of KDs in adults remains uncertain. We identified a limited number of studies which all had small sample sizes. Due to the associated risk of bias and imprecision caused by small study populations, the evidence for the use of KDs was of low to very low certainty. More palatable but related diets, such as the MAD, may have a similar effect on seizure control as the classical KD, but could be associated with fewer adverse effects. This assumption requires more investigation. For people who have drug-resistant epilepsy or who are unsuitable for surgical intervention, KDs remain a valid option. Further research is required, particularly for adults with drug-resistant epilepsy.
Trial registration: ClinicalTrials.gov NCT00836836 NCT01899898 NCT03183076 NCT02708030.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
KM: received PhD funding from The University of Liverpool.
RB: none known
RL: none known
PC: none known
Figures










Update of
-
Ketogenic diets for drug-resistant epilepsy.Cochrane Database Syst Rev. 2018 Nov 7;11(11):CD001903. doi: 10.1002/14651858.CD001903.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Jun 24;6:CD001903. doi: 10.1002/14651858.CD001903.pub5. PMID: 30403286 Free PMC article. Updated.
Comment in
-
What are the effects of ketogenic diets on drug-resistant epilepsy? A Cochrane Review summary with commentary.Dev Med Child Neurol. 2020 Oct;62(10):1121-1123. doi: 10.1111/dmcn.14643. Epub 2020 Aug 18. Dev Med Child Neurol. 2020. PMID: 32808681 No abstract available.
-
Ketogenic Diets for Drug-Resistant Epilepsy.Am Fam Physician. 2021 May 1;103(9):524-525. Am Fam Physician. 2021. PMID: 33929168 No abstract available.
References
References to studies included in this review
Bergqvist 2005 {published data only}
El‐Rashidy 2013 {published data only}
Kim 2016 {published data only}
Kossoff 2007 {published data only}
Kverneland 2018 {published data only}
Lambrechts 2017 {published data only}
-
- IJff DM, Postulart D, Lambrechts DA, Majoie MH, Kinderen RJ, Hendriksen JG, et al. Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: a randomized controlled trial. Epilepsy and Behavior 2016;60:153-7. [DOI: 10.1016/j.yebeh.2016.04.033] [PMID: ] - DOI - PubMed
-
- Wijnen BF, Kinderen RJ, Lambrechts DA, Postulart D, Aldenkamp AP, Majoie MH, et al. Long-term clinical outcomes and economic evaluation of the ketogenic diet versus care as usual in children and adolescents with intractable epilepsy. Epilepsy Research 2017;132:91-9. [DOI: 10.1016/j.eplepsyres.2017.03.002] [PMID: ] - DOI - PubMed
-
- Kinderen RJ, Lambrechts DA, Postulart D, Kessels AG, Hendriksen JG, Aldenkamp AP, et al. Research into the (cost-) effectiveness of the ketogenic diet among children and adolescents with intractable epilepsy: design of a randomized controlled trial. BMC Neurology 2011;11(1):10. [DOI: 10.1186/1471-2377-11-10] [PMID: ] - DOI - PMC - PubMed
-
- Kinderen RJ, Lambrechts DA, Wijnen BF, Postulart D, Aldenkamp AP, Majoie MH, et al. An economic evaluation of the ketogenic diet versus care as usual in children and adolescents with intractable epilepsy: an interim analysis. Epilepsia 2016;57(1):41-50. [DOI: 10.1111/epi.13254] [PMID: ] - DOI - PubMed
McDonald 2018 {published data only}
Neal 2008 {published data only}
-
- Neal E, Chaffe H, Fitzsimmons G, Edwards N, Lawson M, Schwartz R, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy - efficacy and tolerability after 12 months. Epilepsia 2009;50(Suppl 4):86-7, Abstract no: T148. - PubMed
Raju 2011 {published data only}
-
- Raju KN, Gulati S, Kabra M, Agarwala A, Sharma S, Pandey RM, et al. Efficacy of 4:1 (classic) versus 2.5:1 ketogenic ratio diets in refractory epilepsy in young children: a randomized open labeled study. Epilepsy Research 2011;96(1-2):96-100. [DOI: 10.1016/j.eplepsyres.2011.05.005] [PMID: ] - DOI - PubMed
Seo 2007 {published data only}
Sharma 2013 {published data only}
Sharma 2016 {published data only}
-
- Sharma S, Goel S, Jain P, Agarwala A, Aneja S. Evaluation of a simplified modified Atkins diet for use by parents with low levels of literacy in children with refractory epilepsy: a randomized controlled trial. Epilepsy Research 2016;127:152-9. [DOI: 10.1016/j.eplepsyres.2016.09.002] [PMID: ] - DOI - PubMed
References to studies excluded from this review
Dressler 2015 {published data only}
-
- Dressler A, Trimmel-Schwahofer P, Reithofer E, Groppel G, Muhlebner A, Samueli S, et al. The ketogenic diet versus ACTH in the treatment of infantile spasms: a prospective randomised study. European Journal of Paediatric Neurology 2015;19(Suppl 1):S17, Abstract no: OP52-2694.
Freeman 1999 {published data only}
-
- Freeman JM, Vining EP. Seizures decrease rapidly after fasting: preliminary studies of the ketogenic diet. Archives of Pediatrics and Adolescent Medicine 1999;153(9):946-9. [PMID: ] - PubMed
Freeman 2009 {published data only}
-
- Freeman JM, Vining EP, Kossoff EH, Pyzik PL, Ye X, Goodman SN. A blinded, crossover study of the efficacy of the ketogenic diet. Epilepsia 2009;50(2):322-5. [PMID: ] - PubMed
Hemingway 2001 {published data only}
-
- Hemingway C, Freeman JM, Pillas DJ, Pyzik PL. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics 2001;108(4):898-905. [PMID: ] - PubMed
Kang 2011 {published data only}
-
- Kang HC, Lee YJ, Lee JS, Lee EJ, Eom S, You SJ, et al. Comparison of short- versus long-term ketogenic diet for intractable infantile spasms. Epilepsia 2011;52(4):781-7. [PMID: ] - PubMed
NCT03183076 {published data only}
-
- NCT03183076. Efficacy, tolerability and adherence of the Modified Atkins Diet on drug-resistant epilepsy [Efficacy, tolerability and adherence of the Modified Atkins Diet on drug-resistant epilepsy in adult patients]. clinicaltrials.gov/show/nct03183076 (first received 9 June 2017).
Singh 2015 {published data only}
-
- Singh MK, Gulati S, Aggarwal A, Chakrabarty B, Pandey RM, Tripathi M. Efficacy of modified Atkins diet versus ketogenic diet in children with refractory epilepsy aged 1 year to 18 years: a randomised controlled trial. Epilepsia 2015;56(Suppl 1):245, Abstract no: p1000.
Smith 2011 {published data only}
-
- Smith M, Politzer N, Macgarvie D, McAndrews MP, Campo M. Efficacy and tolerability of the Modified Atkins diet in adults with pharmacoresistant epilepsy: a prospective observational study. Epilepsia 2011;52(4):775-80. [PMID: ] - PubMed
References to ongoing studies
CTRI/2015/07/006048 {published data only}
-
- CTRI/2015/07/006048. Modified Atkins diet in adolescence and adults with refractory epilepsy [Efficacy, safety and tolerability of modified Atkins diet in adolescence and adults with drug resistant epilepsy: a randomized controlled trial]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=11330 (first received 27 July 2015 ).
CTRI/2017/12/010898 {published data only}
-
- CTRI/2017/12/010898. Effect of modified Atkins diet when compared to low glycemic index diet in controlling seizures in children with epilepsy who do not respond to conventional anti epileptic drugs [Modified atkins diet versus low glycemic index treatment in drug resistant epilepsy in children: a randomized controlled trial]. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/12/010898 (first received 1 January 2018).
Hulshof 2017 {published data only}
-
- Hulshof H, Jansen F, Braun KP, Carpay H. The modified Atkins diet in patients with refractory epilepsy and intellectual disability: a randomized controlled trial. Epilepsia 2017;58(Suppl 5):S146, Abstract no: p0815.
-
- Hulshof HM, Carpay JA, Jansen FE, Braun KP. The modified Atkins diet in patients with refractory epilepsy and severe intellectual disability - design of a randomized controlled trial. Epilepsia 2014;55(Suppl 2):205, Abstract no: p633.
NCT02708030 {published data only}
-
- Sondhi V, Agarwala A, Chakrabarty B, Jauhari P, Lodha R, Pandey RM, et al. Dietary therapy in epilepsy treatment (Diet-trial): a randomised non-inferiority trial comparing KD, MAD & LGIT for drug resistant epilepsy. Neurology 2018;90(15 Suppl):Abstract no: S35.006.
NCT03464487 {published data only}
-
- NCT03464487. Comparison between efficacy of daily and intermittent low glycemic index therapy diet [Comparison between efficacy of daily and intermittent low glycemic index therapy diet among children with drug resistant epilepsy aged 1-15 years: an open labeled randomized controlled parallel design non-inferiority trial]. clinicaltrials.gov/ct2/show/NCT03464487 (first received 14 March 2018).
NCT03764956 {published data only}
-
- NCT03764956. Comparison of efficacy of LGIT and MAD among children with drug resistant epilepsy [Comparison of efficacy of low glycemic index therapy and modified atkins diet among children with drug resistant epilepsy: a randomized non-inferiority trial]. clinicaltrials.gov/ct2/show/NCT03764956 (first received 5 December 2018).
NCT03807141 {published data only}
-
- NCT03807141. Evaluation of the modified Atkins diet in children with epileptic spasms [Evaluation of the modified Atkins diet in children with epileptic spasms refractory to hormonal therapy: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03807141 (first received 16 January 2019).
Titre‐Johnson 2017 {published data only}
-
- Titre-Johnson S, Schoeler N, Bologun M, Eltze C, Vezyroglou K, Pujar S, et al. Ketogenic diet in the treatment of epilepsy in children under the age of 2 years. Epilepsia 2018;59(Suppl 3):S91-S2, Abstract no: p190.
-
- Titre-Johnson ST, Schoeler NS, Eltze CE, Williams RW, Vezyroglou KV, McCullagh MC, et al. Ketogenic diet in the treatment of epilepsy in children under the age of two years. Developmental medicine and child neurology 2017;59(Suppl 4):119, Abstract no: 202.
Additional references
Atkins 1972
-
- Atkins RC. Dr. Atkins' Diet Revolution: the high calorie way to stay thin forever. Philadelphia, PA: D McKay Co, 1972.
Bough 2007
-
- Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 2007;48(1):43-58. [PMID: ] - PubMed
Chang 2003
-
- Chang BS, Lowenstein DH. Epilepsy. New England Journal of Medicine 2003;349(13):1257-66. [PMID: ] - PubMed
Coppola 2002
-
- Coppola G, Veggiotti P, Cusmai R, Bertoli S, Cardinali S, Dionisi-Vici C, et al. The ketogenic diet in children, adolescents and young adults with refractory epilepsy: an Italian multicentric experience. Epilepsy Research 2002;48(3):221-7. [PMID: ] - PubMed
DiMario 2002
-
- DiMario FJ, Holland J. The Ketogenic diet: a review of the experience at Connecticut Children's Medical Center. Pediatric Neurology 2002;26(4):288-92. [PMID: ] - PubMed
Geyelin 1921
-
- Geyelin HR. Fasting as a method for treating epilepsy. Medical Record 1921;99:1037-9.
GRADEpro 2015 [Computer program]
-
- GRADEpro GDT. Version accessed 15 October 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Granata 2009
Guelpa 1911
-
- Guelpa G, Marie A. La lutte contre l'epilepsie par la desintoxication et par la re-education alimentaire. Revue de Therapie Medico-Chirurgide 1911;78:8-13.
Hauser 1990
-
- Hauser WA, Hesdorffer DC. Epilepsy: Frequency, Causes, and Consequences. New York: Demos Publications, 1990.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hosain 2005
-
- Hosain SA, La Vega-Talbott M, Solomon GE. Ketogenic diet in pediatric epilepsy patients with gastrostomy feeding. Pediatric Neurology 2005;32(2):81-3. [PMID: ] - PubMed
Huttenlocher 1971
-
- Huttenlocher PR, Wilbourn AJ, Signore JM. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971;21(11):1097-103. [PMID: ] - PubMed
Kang 2005
-
- Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia 2005;46(2):272-9. [PMID: ] - PubMed
Kirkham 2010
Kossoff 2005
-
- Kossoff EH, Thiele EA, Pfeifer HH, McGrogan JR, Freeman JM. Tuberous sclerosis complex and the ketogenic diet. Epilepsia 2005;46(10):1684-6. [PMID: ] - PubMed
Kossoff 2008
-
- Kossoff EH, Rowley H, Sinha SR, Vining EP. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia 2008;49(2):316-9. [PMID: ] - PubMed
Lawn 2004
-
- Lawn ND, Bamlet WR, Radhakrishnan K, O'Brien PC, So EL. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology 2004;63(9):1565-70. [PMID: ] - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Merritt 1938
-
- Merritt HH, Putnam TJ. Sodium diphenyl hydantoin in the treatment of convulsive disorders. Journal of the American Medical Association 1938;111:1068-73.
Moesk 2009
-
- Moesk A, Natour H, Neufeld MY, Shiff Y, Vaisman N. Ketogenic diet treatment in adults with refractory epilepsy: a prospective pilot study. Seizure 2009;18(1):30-3. [PMID: ] - PubMed
Moher 2009
NCT00836836
-
- NCT00836836. Modified Atkins diet in childhood epilepsy [Evaluation of the efficacy of the modified Atkins diet in children with refractory epilepsy: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT00836836 (first received 4 February 2009).
Nilsson 1999
-
- Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case control study. Lancet 1999;353(9156):888-93. [PMID: ] - PubMed
O'Connor 2014
-
- O'Connor SE, Ream MA, Richardson C, Mikati MA, Trescher WH, Byler DL, et al. The ketogenic diet for the treatment of pediatric status epilepticus. Pediatric Neurology 2014;50(1):101-3. [PMID: ] - PubMed
Schmidt 2002
-
- Schmidt D. The clinical impact of new antiepileptic drugs after a decade of use in epilepsy. Epilepsy Research 2002;50(1-2):21-32. [PMID: ] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE handbook for grading quality of evidence and strength of recommendations (Updated October 2013). Grade Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Villeneuve 2004
-
- Villeneuve N. Quality-of-life scales for patients with drug-resistant partial epilepsy [Quelles echelles de qualite de vie pour les patients ayant une epilepsie partielle pharmaco-resistante]. Revue Neurologique 2004;160(Spec No 1):5S376-93. [PMID: ] - PubMed
Wilder 1921
-
- Wilder RM. The effects of ketonemia on course of epilepsy. Mayo Clinic Proceedings 1921;2:307-8.
References to other published versions of this review
Levy 2003
Levy 2012
Martin 2016
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

