RNA to the rescue: RNA is one of the most promising targets for drug development given its wide variety of uses
- PMID: 32588530
- PMCID: PMC7332969
- DOI: 10.15252/embr.202051013
RNA to the rescue: RNA is one of the most promising targets for drug development given its wide variety of uses
Abstract
The race for a vaccine against SARS-CoV-2 has accelerated research on RNA-based therapeutics. Beyond vaccines, RNA also shows great potential for cancer therapies.
© 2020 The Author.
Figures


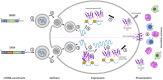
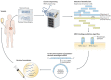
Comment on
-
Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases.ACS Cent Sci. 2020 Mar 25;6(3):315-331. doi: 10.1021/acscentsci.0c00272. Epub 2020 Mar 12. ACS Cent Sci. 2020. PMID: 32226821 Free PMC article. No abstract available.
References
-
- Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du MJ, Hope MJ, Madden TD et al (2019) The Onpattro story and the clinical translation of nanomedicines containing nucleic acid‐based drugs. Nat Nanotechnol 14: 1084–1087 - PubMed
-
- Berkley S (2020) COVID‐19 needs a big science approach. Science 367: 1407 - PubMed
-
- Harries L (2019) It's time for scientists to shout about RNA therapies. Nature 574: S15 - PubMed
-
- John S, Yuzhakov O, Woods A, Deterling J, Hassett K, Shaw CA, Ciaramella G (2018) Multi‐antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell‐mediated immunity. Vaccine 36: 1689–1699 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

