Organization of Bone Sarcoma Care: A Cross-Sectional European Study
- PMID: 32588548
- PMCID: PMC7454217
- DOI: 10.1111/os.12716
Organization of Bone Sarcoma Care: A Cross-Sectional European Study
Abstract
Objective: To assess organization of care in several bone sarcoma centers in Europe affiliated with the European Musculoskeletal Oncology Society (EMSOS) for comparison and to identify potential improvements in organization of care.
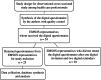
Methods: Data for this observational cross-sectional study was obtained through healthcare professionals affiliated to EMSOS. The authors formulated 10 questions regarding organization of care. The questions were focused on guidance, multidisciplinary decision-making, and data storage. A digital questionnaire was synthesized and included quality control. The digital questionnaire was sent to 54 representative members of EMSOS. We did not receive responses from 29 representative countries (53.7%) after one digital invitation and two digital reminders.
Results: We received data from 25 representatives of bone sarcoma centers from 17 countries across Europe (46.3%). Authorization to perform oncological care in a bone sarcoma center was government issued in 41.2% of cases and based on expertise without governmental influence in 52.9% of cases. In 64.7% of the countries, a national bone tumor guideline regarding for diagnosis and treatment is used in oncological care. A national bone tumor board for extensive case evaluation including classification and advice for treatment is available for 47.1% of the countries. All participating bone sarcoma centers have a mandatory local multidisciplinary meeting before the start of treatment; in 84.0% this meeting takes place once a week. During this multidisciplinary meeting a median of 15 cases (range, 4-40 cases) are discussed. In terms of storage of oncological data, a local registry is used in eight countries (47.1%). A national registry is used in eight countries (47.1%).
Conclusions: A national bone tumor board gives bone sarcoma centers with little adherence the opportunity to gain knowledge from a more experienced team. Centralization of care in a bone sarcoma center is important to lower incidences. The optimal size for a bone sarcoma center in terms of patient adherence is not known at present.
Keywords: Bone sarcoma; Centralization; Organization of care.
© 2020 The Authors. Orthopaedic Surgery published by Chinese Orthopaedic Association and John Wiley & Sons Australia, Ltd.
Figures
References
-
- van Oosterwijk JG, Anninga JK, Gelderblom H, Cleton‐Jansen AM, Bovee JV. Update on targets and novel treatment options for high‐grade osteosarcoma and chondrosarcoma. Hematol Oncol Clin North Am, 2013, 27: 1021–1048. - PubMed
-
- Anninga JK, Gelderblom H, Fiocco M, et al Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer, 2011, 47: 2431–2445. - PubMed
-
- Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with Ewing's sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol, 2015. Apr, 39: 189–195. - PubMed
-
- Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high‐grade osteosarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol, 2015, 39: 593–599. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

