Outcomes of Hemospray therapy in the treatment of intraprocedural upper gastrointestinal bleeding post-endoscopic therapy
- PMID: 32588788
- PMCID: PMC7724526
- DOI: 10.1177/2050640620938549
Outcomes of Hemospray therapy in the treatment of intraprocedural upper gastrointestinal bleeding post-endoscopic therapy
Abstract
Introduction: With increasing advances in minimally invasive endoscopic therapies and endoscopic resection techniques for luminal disease, there is an increased risk of post-procedure bleeding. This can contribute to significant burden on patient's quality of life and health resources when reintervention is required. Hemospray (Cook Medical, North Carolina, USA) is a novel haemostatic powder licensed for gastrointestinal bleeding. The aim of this single-arm, prospective, non-randomised multicentre international study is to look at outcomes in patients with upper gastrointestinal bleeds following elective endoscopic therapy treated with Hemospray to achieve haemostasis.
Methods: Data was prospectively collected on the use of Hemospray from 16 centres (January 2016-November 2019). Hemospray was used during the presence of progressive intraprocedural bleeding post-endoscopic therapy as a monotherapy, dual therapy with standard haemostatic techniques or rescue therapy once standard methods had failed. Haemostasis was defined as the cessation of bleeding within 5 min of the application of Hemospray. Re-bleeding was defined as a sustained drop in haemoglobin (>2 g/l), haematemesis or melaena with haemodynamic instability after the index endoscopy.
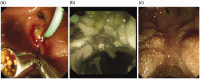
Results: A total of 73 patients were analysed with bleeding post-endoscopic therapy. The median Blatchford score at baseline was five (interquartile range 0-9). The median Rockall score was six (interquartile range 5-7). Immediate haemostasis following the application of Hemospray was achieved in 73/73 (100%) of patients. Two out of 57 (4%) had a re-bleed post-Hemospray, one was following oesophageal endoscopic mucosal resection and the other post-duodenal endoscopic mucosal resection. Both patients had a repeat endoscopy and therapy within 24 h. Re-bleeding data was missing for 16 patients, and mortality data was missing for 14 patients. There were no adverse events recorded in association with the use of Hemospray.
Conclusion: Hemospray is safe and effective in achieving immediate haemostasis following uncontrolled and progressive intraprocedural blood loss post-endoscopic therapy, with a low re-bleed rate.
Keywords: Hemospray; TC-325; endoscopy; post-endotherapy bleeding; upper gastro-intestinal bleeding.
Conflict of interest statement
Figures


Similar articles
-
Outcomes from an international multicenter registry of patients with acute gastrointestinal bleeding undergoing endoscopic treatment with Hemospray.Dig Endosc. 2020 Jan;32(1):96-105. doi: 10.1111/den.13502. Epub 2019 Aug 30. Dig Endosc. 2020. PMID: 31365756
-
Hemostatic powder TC-325 treatment of malignancy-related upper gastrointestinal bleeds: International registry outcomes.J Gastroenterol Hepatol. 2021 Nov;36(11):3027-3032. doi: 10.1111/jgh.15579. Epub 2021 Jun 28. J Gastroenterol Hepatol. 2021. PMID: 34132412 Free PMC article.
-
Hemospray for treatment of acute bleeding due to upper gastrointestinal tumours.Dig Liver Dis. 2017 May;49(5):514-517. doi: 10.1016/j.dld.2016.12.012. Epub 2016 Dec 21. Dig Liver Dis. 2017. PMID: 28065526
-
Use of Hemospray in the treatment of patients with acute UGIB - short review.J Med Life. 2013 Jun 15;6(2):117-9. Epub 2013 Jun 25. J Med Life. 2013. PMID: 23904868 Free PMC article. Review.
-
Endoscopic management of intraprocedural bleeding during endoscopic interventions.Best Pract Res Clin Gastroenterol. 2024 Mar;69:101912. doi: 10.1016/j.bpg.2024.101912. Epub 2024 Apr 7. Best Pract Res Clin Gastroenterol. 2024. PMID: 38749579 Review.
Cited by
-
Topical hemostatic agents in the management of upper gastrointestinal bleeding: a meta-analysis.Endosc Int Open. 2023 Apr 24;11(4):E368-E385. doi: 10.1055/a-1984-6895. eCollection 2023 Apr. Endosc Int Open. 2023. PMID: 37102185 Free PMC article.
-
Hemospray® (hemostatic powder TC-325) as monotherapy for acute gastrointestinal bleeding: a multicenter prospective study.Ann Gastroenterol. 2024 Jul-Aug;37(4):418-426. doi: 10.20524/aog.2024.0897. Epub 2024 Jun 20. Ann Gastroenterol. 2024. PMID: 38974074 Free PMC article.
-
UEGWeek: The modern School of Athens.United European Gastroenterol J. 2021 Sep;9(7):877-882. doi: 10.1002/ueg2.12149. United European Gastroenterol J. 2021. PMID: 35099123 Free PMC article. No abstract available.
-
Propensity score matching analysis to evaluate efficacy of polyethylene oxide adhesive on preventing delayed bleeding after gastric endoscopic submucosal dissection.Sci Rep. 2022 Mar 16;12(1):4538. doi: 10.1038/s41598-022-08499-0. Sci Rep. 2022. PMID: 35297400 Free PMC article.
-
Closure of Mucosal Defects Using Endoscopic Suturing Following Endoscopic Submucosal Dissection: A Single-Center Experience.Tech Innov Gastrointest Endosc. 2023;25(1):46-51. doi: 10.1016/j.tige.2022.11.002. Epub 2022 Nov 12. Tech Innov Gastrointest Endosc. 2023. PMID: 37799128 Free PMC article.
References
-
- Lanas A, Aabakken L, Fonseca et al. Clinical predictors of poor outcomes among patients with nonvariceal upper gastrointestinal bleeding in Europe. Aliment Pharmacol Ther 2011; 33: 1225–1233. - PubMed
-
- Bakman Y, Freeman ML. Update on biliary and pancreatic sphincterotomy. Curr Opin Gastroenterol 2012; 28: 420–426. - PubMed
-
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD study group multicentre study. Gastrointest Endosc 2009; 69: 1228–1235. - PubMed
-
- Isomoto H, Shikuwa S, Yamaguchi Net al. Endoscopic submucosal dissection for early gastric cancer: A large-scale feasibility study. Gut 2009; 58: 331–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

