Genomic characterization of Salmonella Uzaramo for human invasive infection
- PMID: 32589568
- PMCID: PMC7478631
- DOI: 10.1099/mgen.0.000401
Genomic characterization of Salmonella Uzaramo for human invasive infection
Abstract
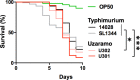
Salmonella is composed of a wide variety of serovars, causing human self-limited gastrointestinal illnesses or invasive infections. Invasive non-typhoidal Salmonella (iNTS) is well documented, with high mortality for children and immunocompromised adults in sub-Saharan Africa and has recently been reported in Southeast Asia. However, iNTS in China remains unknown. In May 2019, a case of invasive infection caused by Salmonella enterica serovar Uzaramo (S. Uzaramo) was reported for the first time in China. Phylogenomic analysis was performed by genomic sequencing the available contextualized isolates, which separated the two Chinese strains into different sublineages. Both phenotypic and genomic characterization demonstrated that the S. Uzaramo isolates showed in general low antimicrobial resistance potential, except one isolated from lake-water in China. Additional comparative genomic analysis and Caenorhabditis elegans killing assays suggested a unique combination of virulence factors, including typhoid toxin and tcf fimbrial adhesin, which might play a role in the invasive infection. This study highlights that the transparency of global surveillance genomic data could accelerate understanding of virulence and antimicrobial resistance makeup of a previously unknown threat.
Keywords: Salmonella Uzaramo; bloodstream infection; foodborne transmission; sublineage; whole genome sequencing.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

References
-
- Pan H, Li X, Fang W, Yue M. Analysis of major human and foodborne pathogens and their resistance to antimicrobials in the USA in the past two decades: Implications for surveillance and control of antimicrobial resistance in China. Journal of Zhejiang University. 2019;44:237–246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

