Layer 4 Gates Plasticity in Visual Cortex Independent of a Canonical Microcircuit
- PMID: 32589913
- PMCID: PMC7919382
- DOI: 10.1016/j.cub.2020.05.067
Layer 4 Gates Plasticity in Visual Cortex Independent of a Canonical Microcircuit
Erratum in
-
Layer 4 Gates Plasticity in Visual Cortex Independent of a Canonical Microcircuit.Curr Biol. 2023 Jun 19;33(12):2586. doi: 10.1016/j.cub.2023.05.038. Curr Biol. 2023. PMID: 37339587 Free PMC article. No abstract available.
Abstract
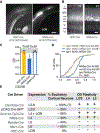
Disrupting binocular vision during a developmental critical period can yield enduring changes to ocular dominance (OD) in primary visual cortex (V1). Here we investigated how this experience-dependent plasticity is coordinated within the laminar circuitry of V1 by deleting separately in each cortical layer (L) a gene required to close the critical period, nogo-66 receptor (ngr1). Deleting ngr1 in excitatory neurons in L4, but not in L2/3, L5, or L6, prevented closure of the critical period, and adult mice remained sensitive to brief monocular deprivation. Intracortical disinhibition, but not thalamocortical disinhibition, accompanied this OD plasticity. Both juvenile wild-type mice and adult mice lacking ngr1 in L4 displayed OD plasticity that advanced more rapidly L4 than L2/3 or L5. Interestingly, blocking OD plasticity in L2/3 with the drug AM-251 did not impair OD plasticity in L5. We propose that L4 restricts disinhibition and gates OD plasticity independent of a canonical cortical microcircuit.
Keywords: amblyopia; cortical circuit; critical period; experience-dependent plasticity; myelin; ocular dominance; reticulon receptor; visual cortex.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



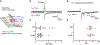
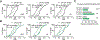
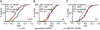
References
-
- Wiesel TN, and Hubel DH (1963). Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J. Neurophysiol 26, 1003–1017. - PubMed
-
- Hubel DH, Wiesel TN, and LeVay S. (1977). Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci 278, 377–409. - PubMed
-
- Drager UC (1978). Observations on monocular deprivation in mice. J. Neurophysiol 41, 28–42. - PubMed
-
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, and Maffei L. (1994). Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular deprivation. Vision Res. 34, 709–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

