Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the Dynamic Responses to Exercise
- PMID: 32589957
- PMCID: PMC8800485
- DOI: 10.1016/j.cell.2020.06.004
Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the Dynamic Responses to Exercise
Abstract
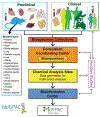
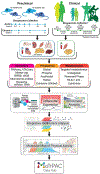
Exercise provides a robust physiological stimulus that evokes cross-talk among multiple tissues that when repeated regularly (i.e., training) improves physiological capacity, benefits numerous organ systems, and decreases the risk for premature mortality. However, a gap remains in identifying the detailed molecular signals induced by exercise that benefits health and prevents disease. The Molecular Transducers of Physical Activity Consortium (MoTrPAC) was established to address this gap and generate a molecular map of exercise. Preclinical and clinical studies will examine the systemic effects of endurance and resistance exercise across a range of ages and fitness levels by molecular probing of multiple tissues before and after acute and chronic exercise. From this multi-omic and bioinformatic analysis, a molecular map of exercise will be established. Altogether, MoTrPAC will provide a public database that is expected to enhance our understanding of the health benefits of exercise and to provide insight into how physical activity mitigates disease.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
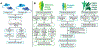

References
-
- Barrachina MN, Calderón-Cruz B, Fernandez-Rocca L, and García Á (2019). Application of Extracellular Vesicles Proteomics to Cardiovascular Disease: Guidelines, Data Analysis, and Future Perspectives. Proteomics 19, e1800247. - PubMed
-
- Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, and Zierath JR (2012). Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 15, 405–411. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 AG055135/AG/NIA NIH HHS/United States
- U01 AR071158/AR/NIAMS NIH HHS/United States
- U01 AG055137/AG/NIA NIH HHS/United States
- U01 AR071130/AR/NIAMS NIH HHS/United States
- U01 AR071133/AR/NIAMS NIH HHS/United States
- U24 DK112340/DK/NIDDK NIH HHS/United States
- U24 EB023674/EB/NIBIB NIH HHS/United States
- U24 DK112341/DK/NIDDK NIH HHS/United States
- U24 DK112326/DK/NIDDK NIH HHS/United States
- U24 OD026629/OD/NIH HHS/United States
- U24 DK112342/DK/NIDDK NIH HHS/United States
- U24 DK112331/DK/NIDDK NIH HHS/United States
- U24 AR071113/AR/NIAMS NIH HHS/United States
- U01 AR071160/AR/NIAMS NIH HHS/United States
- U24 DK112348/DK/NIDDK NIH HHS/United States
- U24 OD036598/OD/NIH HHS/United States
- U01 AG055133/AG/NIA NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- U01 AR071128/AR/NIAMS NIH HHS/United States
- P30 AG028740/AG/NIA NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- U01 AR071124/AR/NIAMS NIH HHS/United States
- R01 GM070335/GM/NIGMS NIH HHS/United States
- U24 DK112349/DK/NIDDK NIH HHS/United States
- U01 AR071150/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

