Implementing newborn screening for sickle cell disease as part of immunisation programmes in Nigeria: a feasibility study
- PMID: 32589979
- PMCID: PMC7322555
- DOI: 10.1016/S2352-3026(20)30143-5
Implementing newborn screening for sickle cell disease as part of immunisation programmes in Nigeria: a feasibility study
Erratum in
-
Correction to Lancet Haematol 2020; 7: e534-40.Lancet Haematol. 2020 Aug;7(8):e560. doi: 10.1016/S2352-3026(20)30230-1. Lancet Haematol. 2020. PMID: 32735834 Free PMC article. No abstract available.
Abstract
Background: Sickle cell disease is highly prevalent in sub-Saharan Africa, where it accounts for substantial morbidity and mortality. Newborn screening is paramount for early diagnosis and enrolment of affected children into a comprehensive care programme. Up to now, this strategy has been greatly impaired in resource-poor countries, because screening methods are technologically and financially intensive; affordable, reliable, and accurate methods are needed. We aimed to test the feasibility of implementing a sickle cell disease screening programme using innovative point-of-care test devices into existing immunisation programmes in primary health-care settings.
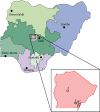
Methods: Building on a routine immunisation programme and using existing facilities and staff, we did a prospective feasibility study at five primary health-care centres within Gwagwalada Area Council, Abuja, Nigeria. We systematically screened for sickle cell disease consecutive newborn babies and infants younger than 9 months who presented to immunisation clinics at these five centres, using an ELISA-based point-of care test (HemoTypeSC). A subgroup of consecutive babies who presented to immunisation clinics at the primary health-care centres, whose mothers gave consent, were tested by the HemoTypeSC point-of-care test alongside a different immunoassay-based point-of-care test (SickleSCAN) and the gold standard test, high-performance liquid chromatography (HPLC).
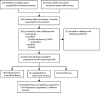
Findings: Between July 14, 2017, and Sept 3, 2019, 3603 newborn babies and infants who presented for immunisation were screened for sickle cell disease at five primary health-care centres using the ELISA-based point-of-care test. We identified 51 (1%) children with sickle cell anaemia (HbSS), four (<1%) heterozygous for HbS and HbC (HbSC), 740 (21%) with sickle cell trait (HbAS), 34 (1%) heterozygous for HbA and HbC (HbAC), and 2774 (77%) with normal haemoglobin (HbAA). Of the 55 babies and infants with confirmed sickle cell disease, 41 (75%) were enrolled into a programme for free folic acid and penicillin, of whom 36 (88%) completed three visits over 9 months (median follow-up 226 days [IQR 198-357]). The head-to-head comparison between the two point-of-care tests and HPLC showed concordance between the three testing methods in screening 313 newborn babies, with a specificity of 100% with HemoTypeSC, 100% with SickleSCAN, and 100% by HPLC, and a sensitivity of 100% with HemoTypeSC, 100% with SickleSCAN, and 100% by HPLC.
Interpretation: Our pilot study shows that the integration of newborn screening into existing primary health-care immunisation programmes is feasible and can rapidly be implemented with limited resources. Point-of-care tests are reliable and accurate in newborn screening for sickle cell disease. This feasibility study bodes well for the care of patients with sickle cell disease in resource-poor countries.
Funding: Doris Duke Charitable Foundation, Imperial College London Wellcome Trust Centre for Global Health Research, and Richard and Susan Kiphart Family Foundation.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access Article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Screening infants for sickle cell disease in sub-Saharan Africa: starting the journey to a sustainable model in primary care.Lancet Haematol. 2020 Jul;7(7):e503-e504. doi: 10.1016/S2352-3026(20)30180-0. Lancet Haematol. 2020. PMID: 32589971 No abstract available.
-
Communicating sickle cell disease point-of-care testing results.Lancet Haematol. 2020 Oct;7(10):e708-e709. doi: 10.1016/S2352-3026(20)30280-5. Lancet Haematol. 2020. PMID: 32976746 No abstract available.
References
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311–323. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

