Role of neuronal nitric oxide synthase on cardiovascular functions in physiological and pathophysiological states
- PMID: 32590118
- PMCID: PMC7375925
- DOI: 10.1016/j.niox.2020.06.004
Role of neuronal nitric oxide synthase on cardiovascular functions in physiological and pathophysiological states
Abstract
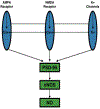
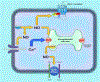
This review describes and summarizes the role of neuronal nitric oxide synthase (nNOS) on the central nervous system, particularly on brain regions such as the ventrolateral medulla (VLM) and the periaqueductal gray matter (PAG), and on blood vessels and the heart that are involved in the regulation and control of the cardiovascular system (CVS). Furthermore, we shall also review the functional aspects of nNOS during several physiological, pathophysiological, and clinical conditions such as exercise, pain, cerebral vascular accidents or stroke and hypertension. For example, during stroke, a cascade of molecular, neurochemical, and cellular changes occur that affect the nervous system as elicited by generation of free radicals and nitric oxide (NO) from vulnerable neurons, peroxide formation, superoxides, apoptosis, and the differential activation of three isoforms of nitric oxide synthases (NOSs), and can exert profound effects on the CVS. Neuronal NOS is one of the three isoforms of NOSs, the others being endothelial (eNOS) and inducible (iNOS) enzymes. Neuronal NOS is a critical homeostatic component of the CVS and plays an important role in regulation of different systems and disease process including nociception. The functional and physiological roles of NO and nNOS are described at the beginning of this review. We also elaborate the structure, gene, domain, and regulation of the nNOS protein. Both inhibitory and excitatory role of nNOS on the sympathetic autonomic nervous system (SANS) and parasympathetic autonomic nervous system (PANS) as mediated via different neurotransmitters/signal transduction processes will be explored, particularly its effects on the CVS. Because the VLM plays a crucial function in cardiovascular homeostatic mechanisms, the neuroanatomy and cardiovascular regulation of the VLM will be discussed in conjunction with the actions of nNOS. Thereafter, we shall discuss the up-to-date developments that are related to the interaction between nNOS and cardiovascular diseases such as hypertension and stroke. Finally, we shall focus on the role of nNOS, particularly within the PAG in cardiovascular regulation and neurotransmission during different types of pain stimulus. Overall, this review focuses on our current understanding of the nNOS protein, and provides further insights on how nNOS modulates, regulates, and controls cardiovascular function during both physiological activity such as exercise, and pathophysiological conditions such as stroke and hypertension.
Keywords: Antioxidants; Arteriosclerosis; Autonomic nervous system; Baroreceptor; Blood pressure; Cerebral vascular accident; Endothelial cells; Exercise; Exercise pressor reflex; GABA; Glutamate; Heart rate; Hypertension; Hypothalamus; Nociception; Periaqueductal gray matter; Reactive oxygen species; Stroke; Thrombus; Ventrolateral medulla.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures




References
-
- Morris SM Jr., Enzymes of arginine metabolism, J Nutr 134 (2004) 2743S-2747S; discussion 2765S-2767S. - PubMed
-
- Tapiero H, The 2004 French Medical Academy award to professor Georges Mathe, Biomed Pharmacother 59 (2005) 63. - PubMed
-
- Kishimoto J, Spurr N, Liao M, Lizhi L, Emson P, Xu W, Localization of brain nitric oxide synthase (NOS) to human chromosome 12, Genomics 14 (1992) 802–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

