Budesonide versus systemic corticosteroids in IgA Nephropathy: A retrospective, propensity-matched comparison
- PMID: 32590815
- PMCID: PMC7329020
- DOI: 10.1097/MD.0000000000021000
Budesonide versus systemic corticosteroids in IgA Nephropathy: A retrospective, propensity-matched comparison
Abstract
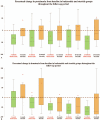
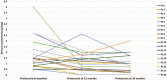
IgA Nephropathy (IgAN) is characterized by mesangial deposition of dominant, polymeric, galactose-deficient IgA1 molecules of gut-associated lymphoid tissue origin. We sought to evaluate the efficacy of targeting the mucosal immune system dysregulation underlying IgAN pathogenesis with a pH-modified formulation of budesonide with a maximum release of active compound in the distal ileum and proximal colon.We did a retrospective study evaluating the efficacy of budesonide (Budenofalk) in the treatment of IgAN. From a retrospective cohort of 143 patients with IgAN followed in our department we identified 21 patients that received treatment with budesonide. These patients received budesonide at a dose of 9 mg/d in the first 12 months, followed by a dose reduction to 3 mg/d for the subsequent period. Only patients that received a 24-month treatment with budesonide were included in the analysis (n = 18). We matched the budesonide-treated cohort to 18 patients with IgAN treated with systemic steroids from the same retrospective cohort. Efficacy was measured as change in proteinuria, hematuria and estimated glomerular filtration rate over a 24-month period.Treatment with budesonide was associated with a 24-month renal function decline of -0.22 (95%CI, -8.2 to 7.8) ml/min/1.73m, compared to -5.89 (95%CI, -12.2 to 0.4) ml/min/1.73m in the corticosteroid treatment group (p = 0.44, for between group difference). The median reduction in proteinuria at 24-month was 45% (interquartile range [IQR]: -79%; -22%) in the budesonide group and 11% (IQR: -39%; 43%) in the corticosteroid group, respectively (P = .009, for between group difference). The median reduction in hematuria at 24-month was 72% (IQR: -90%; -45%) in the budesonide group and 73% (IQR: -85%; 18%) in the corticosteroid group, respectively (P = .22, for between group difference). Treatment with budesonide was well tolerated with minimal side effects.Budesonide (Budenofalk) was effective in the treatment of patients with IgAN at high-risk of progression in terms of reducing proteinuria, hematuria and preserving renal function over 24 months of therapy.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012;2:1–274.
-
- Rauen T, Eitner F, Fitzner C, et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015;373:2225–36. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous