Xpert Mycobacterium tuberculosis/Rifampicin-Detected Rifampicin Resistance is a Suboptimal Surrogate for Multidrug-resistant Tuberculosis in Eastern Democratic Republic of the Congo: Diagnostic and Clinical Implications
- PMID: 32590841
- PMCID: PMC8282324
- DOI: 10.1093/cid/ciaa873
Xpert Mycobacterium tuberculosis/Rifampicin-Detected Rifampicin Resistance is a Suboptimal Surrogate for Multidrug-resistant Tuberculosis in Eastern Democratic Republic of the Congo: Diagnostic and Clinical Implications
Abstract
Background: Rifampicin (RIF) resistance is highly correlated with isoniazid (INH) resistance and used as proxy for multidrug-resistant tuberculosis (MDR-TB). Using MTBDRplus as a comparator, we evaluated the predictive value of Xpert MTB/RIF (Xpert)-detected RIF resistance for MDR-TB in eastern Democratic Republic of the Congo (DRC).
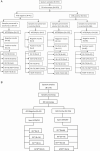
Methods: We conducted a cross-sectional study involving data from new or retreatment pulmonary adult TB cases evaluated between July 2013 and December 2016. Separate, paired sputa for smear microscopy and MTBDRplus were collected. Xpert testing was performed subject to the availability of Xpert cartridges on sample remnants after microscopy.
Results: Among 353 patients, 193 (54.7%) were previously treated and 224 (63.5%) were MTBDRplus TB positive. Of the 224, 43 (19.2%) were RIF monoresistant, 11 (4.9%) were INH monoresistant, 53 (23.7%) had MDR-TB, and 117 (52.2%) were RIF and INH susceptible. Overall, among the 96 samples detected by MTBDRplus as RIF resistant, 53 (55.2%) had MDR-TB. Xpert testing was performed in 179 (50.7%) specimens; among these, 163 (91.1%) were TB positive and 73 (44.8%) RIF resistant. Only 45/73 (61.6%) Xpert-identified RIF-resistant isolates had concomitant MTBDRplus-detected INH resistance. Xpert had a sensitivity of 100.0% (95% CI, 92.1-100.0) for detecting RIF resistance but a positive-predictive value of only 61.6% (95% CI, 49.5-72.8) for MDR-TB. The most frequent mutations associated with RIF and INH resistance were S531L and S315T1, respectively.
Conclusions: In this high-risk MDR-TB study population, Xpert had low positive-predictive value for the presence of MDR-TB. Comprehensive resistance testing for both INH and RIF should be performed in this setting.
Keywords: inhA mutations; rpoB mutations; GenoType MTBDRplus assay; DRC; drug resistance.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- World Health Organization. Geneva: Global Tuberculosis Report 2019. WHO Press; 2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-en.... Acessed 25 January 2020.
-
- World Health Organization. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). Geneva, Switzerland: World Health Organization, 2018. Available at: http://www.who.int/tb/publications/2018/WHO_RapidCommunicationMDRTB.pdf?.... Accessed 21 November 2018.
-
- World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment [Internet]. WHO Press (Geneva) 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-en...; Accessed 12 May 2020. - PubMed
-
- Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:223–34. - PubMed

