A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection
- PMID: 32591393
- PMCID: PMC7566074
- DOI: 10.4049/jimmunol.2000583
A Potently Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for millions of infections and hundreds of thousands of deaths globally. There are no widely available licensed therapeutics against SARS-CoV-2, highlighting an urgent need for effective interventions. The virus enters host cells through binding of a receptor-binding domain within its trimeric spike glycoprotein to human angiotensin-converting enzyme 2. In this article, we describe the generation and characterization of a panel of murine mAbs directed against the receptor-binding domain. One mAb, 2B04, neutralized wild-type SARS-CoV-2 in vitro with remarkable potency (half-maximal inhibitory concentration of <2 ng/ml). In a murine model of SARS-CoV-2 infection, 2B04 protected challenged animals from weight loss, reduced lung viral load, and blocked systemic dissemination. Thus, 2B04 is a promising candidate for an effective antiviral that can be used to prevent SARS-CoV-2 infection.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures


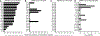
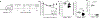
References
-
- Drosten C, Günther S, Preiser W, van der Werf S, Brodt H-R, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RAM, Berger A, Burguière A-M, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra J-C, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk H-D, Osterhaus ADME, Schmitz H, and Doerr HW. 2003. Identification of a Novel Coronavirus in Patients with Severe Acute Respiratory Syndrome. N. Engl. J. Med 348: 1967–1976. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling A-E, Humphrey CD, Shieh W-J, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang J-Y, Cox N, Hughes JM, LeDuc JW, Bellini WJ, and Anderson LJ. 2003. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med 348: 1953–1966. - PubMed
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, and Fouchier RAM. 2012. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med 367: 1814–1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI139813/AI/NIAID NIH HHS/United States
- R01 AI157155/AI/NIAID NIH HHS/United States
- U01 AI141990/AI/NIAID NIH HHS/United States
- 75N93019C00062/AO/NIAID NIH HHS/United States
- T32 CA009547/CA/NCI NIH HHS/United States
- R01 AI104739/AI/NIAID NIH HHS/United States
- T32 AI007163/AI/NIAID NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- HHSN272201700060C/AI/NIAID NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- 75N93019C00062/AI/NIAID NIH HHS/United States
- HHSN272201400008C/AI/NIAID NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- F30 AI152327/AI/NIAID NIH HHS/United States
- R01 AI127828/AI/NIAID NIH HHS/United States
- 75N93019C00051/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

