Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome
- PMID: 32591408
- PMCID: PMC7319493
- DOI: 10.1126/sciimmunol.abd2071
Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome
Abstract
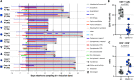
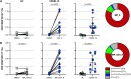
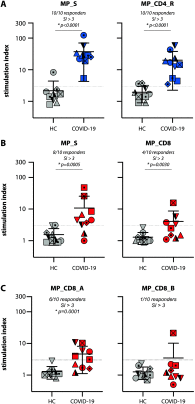
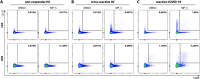
SARS-CoV-2 has been identified as the causative agent of a global outbreak of respiratory tract disease (COVID-19). In some patients the infection results in moderate to severe acute respiratory distress syndrome (ARDS), requiring invasive mechanical ventilation. High serum levels of IL-6, IL-10 and an immune hyperresponsiveness referred to as a 'cytokine storm' have been associated with poor clinical outcome. Despite the large numbers of COVID-19 cases and deaths, information on the phenotype and kinetics of SARS-CoV-2-specific T cells is limited. Here, we studied 10 COVID-19 patients who required admission to an intensive care unit and detected SARS-CoV-2-specific CD4+ and CD8+ T cells in 10 out of 10 and 8 out of 10 patients, respectively. We also detected low levels of SARS-CoV-2-reactive T cells in 2 out of 10 healthy controls not previously exposed to SARS-CoV-2, which is indicative of cross-reactivity due to past infection with 'common cold' coronaviruses. The strongest T-cell responses were directed to the spike (S) surface glycoprotein, and SARS-CoV-2-specific T cells predominantly produced effector and Th1 cytokines, although Th2 and Th17 cytokines were also detected. Furthermore, we studied T-cell kinetics and showed that SARS-CoV-2-specific T cells are present relatively early and increase over time. Collectively, these data shed light on the potential variations in T-cell responses as a function of disease severity, an issue that is key to understanding the potential role of immunopathology in the disease, and also inform vaccine design and evaluation.
Copyright © 2020, American Association for the Advancement of Science.
Figures



References
-
- Chan J. F., Yuan S., Kok K.-H., To K. K.-W., Chu H., Yang J., Xing F., Liu J., Yip C. C.-Y., Poon R. W.-S., Tsoi H.-W., Lo S. K.-F., Chan K.-H., Poon V. K.-M., Chan W.-M., Ip J. D., Cai J.-P., Cheng V. C.-C., Chen H., Hui C. K.-M., Yuen K.-Y., A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 395, 514–523 (2020). 10.1016/S0140-6736(20)30154-9 - DOI - PMC - PubMed
-
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). 10.1016/S0140-6736(20)30183-5 - DOI - PMC - PubMed
-
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q., Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130, 2620–2629 (2020). 10.1172/JCI137244 - DOI - PMC - PubMed
-
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S., Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 8, 420–422 (2020). 10.1016/S2213-2600(20)30076-X - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

