A randomized, double-blind, active placebo-controlled study of efficacy, safety, and durability of repeated vs single subanesthetic ketamine for treatment-resistant depression
- PMID: 32591498
- PMCID: PMC7319954
- DOI: 10.1038/s41398-020-00897-0
A randomized, double-blind, active placebo-controlled study of efficacy, safety, and durability of repeated vs single subanesthetic ketamine for treatment-resistant depression
Abstract
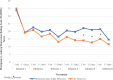
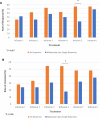
The strategy of repeated ketamine in open-label and saline-control studies of treatment-resistant depression suggested greater antidepressant response beyond a single ketamine. However, consensus guideline stated the lack of evidence to support frequent ketamine administration. We compared the efficacy and safety of single vs. six repeated ketamine using midazolam as active placebo. Subjects received either six ketamine or five midazolam followed by a single ketamine during 12 days followed by up to 6-month post-treatment period. The primary end point was the change from baseline in the Montgomery-Åsberg Depression Rating Scale (MADRS) score at 24 h after the last infusion. Fifty-four subjects completed all six infusions. For the primary outcome measure, there was no significant difference in change of MADRS scores between six ketamine group and single ketamine group at 24 h post-last infusion. Repeated ketamine showed greater antidepressant efficacy compared to midazolam after five infusions before receiving single ketamine infusion. Remission and response favored the six ketamine after infusion 4 and 5, respectively, compared to midazolam before receiving single ketamine infusion. For those who responded, the median time-to-relapse was nominally but not statistically different (2 and 6 weeks for the single and six ketamine group, respectively). Repeated infusions were relatively well-tolerated. Repeated ketamine showed greater antidepressant efficacy to midazolam after five infusions but fell short of significance when compared to add-on single ketamine to midazolam at the end of 2 weeks. Increasing knowledge on the mechanism of ketamine should drive future studies on the optimal balance of dosing ketamine for maximum antidepressant efficacy with minimum exposure.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Singh, J. B. et al. A Double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am. J. Psychiatry10.1176/appi.ajp.2016.16010037 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

