Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing
- PMID: 32591530
- PMCID: PMC7320157
- DOI: 10.1038/s41467-020-17029-3
Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing
Abstract
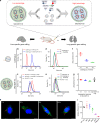
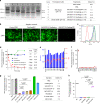
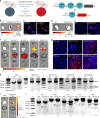
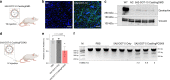
CRISPR-Cas9 has emerged as a powerful technology that relies on Cas9/sgRNA ribonucleoprotein complexes (RNPs) to target and edit DNA. However, many therapeutic targets cannot currently be accessed due to the lack of carriers that can deliver RNPs systemically. Here, we report a generalizable methodology that allows engineering of modified lipid nanoparticles to efficiently deliver RNPs into cells and edit tissues including muscle, brain, liver, and lungs. Intravenous injection facilitated tissue-specific, multiplexed editing of six genes in mouse lungs. High carrier potency was leveraged to create organ-specific cancer models in livers and lungs of mice though facile knockout of multiple genes. The developed carriers were also able to deliver RNPs to restore dystrophin expression in DMD mice and significantly decrease serum PCSK9 level in C57BL/6 mice. Application of this generalizable strategy will facilitate broad nanoparticle development for a variety of disease targets amenable to protein delivery and precise gene correction approaches.
Conflict of interest statement
D.J.S., Q.C., T.W., and the Reagents of the University of Texas System have filed patent applications (PCT/US19/49552 and PCT/US19/49565) on the compositions and methods for organ-specific delivery of nucleic acids. D.J.S. is a co-founder of, and consultant to, ReCode Therapeutics, which has licensed intellectual property from UT Southwestern. Y.-L.M. is currently an employee of Vertex Pharmaceuticals. E.N.O. is a consultant for Vertex Pharmaceuticals.
Figures

References
-
- Wang HX, et al. CRISPR/Cas9-based genome editing for disease modeling and therapy: challenges and opportunities for nonviral delivery. Chem. Rev. 2017;117:9874–9906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

