Individual differences in local functional brain connectivity affect TMS effects on behavior
- PMID: 32591568
- PMCID: PMC7320140
- DOI: 10.1038/s41598-020-67162-8
Individual differences in local functional brain connectivity affect TMS effects on behavior
Abstract
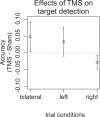
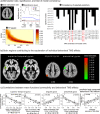
Behavioral effects of transcranial magnetic stimulation (TMS) often show substantial differences between subjects. One factor that might contribute to these inter-individual differences is the interaction of current brain states with the effects of local brain network perturbation. The aim of the current study was to identify brain regions whose connectivity before and following right parietal perturbation affects individual behavioral effects during a visuospatial target detection task. 20 subjects participated in an fMRI experiment where their brain hemodynamic response was measured during resting state, and then during a visuospatial target detection task following 1 Hz rTMS and sham stimulation. To select a parsimonious set of associated brain regions, an elastic net analysis was used in combination with a whole-brain voxel-wise functional connectivity analysis. TMS-induced changes in accuracy were significantly correlated with the pattern of functional connectivity during the task state following TMS. The functional connectivity of the left superior temporal, angular, and precentral gyri was identified as key explanatory variable for the individual behavioral TMS effects. Our results suggest that the brain must reach an appropriate state in which right parietal TMS can induce improvements in visual target detection. The ability to reach this state appears to vary between individuals.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

