Gabapentin-induced drug-seeking-like behavior: a potential role for the dopaminergic system
- PMID: 32591630
- PMCID: PMC7320158
- DOI: 10.1038/s41598-020-67318-6
Gabapentin-induced drug-seeking-like behavior: a potential role for the dopaminergic system
Abstract
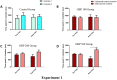
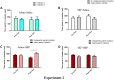
Drugs of abuse represent a growing public health crisis. Accumulating evidence indicates that gabapentin (GBP), a prescription drug, is prone to misuse, abuse, withdrawal, and dependence. Commonly, drugs of abuse modulate the dopaminergic system to induce addiction. In this study, we used the conditioned place preference (CPP) model to investigate the involvement of the dopamine 1 (D1) receptor on the reward and reinforcement behavior of GBP. Under a CPP paradigm, male BALB/c mice were intraperitoneally injected either saline or 100, 200, or 300 mg/kg of GBP and confined to the injection-paired chamber for 30 min. In the pre-conditioning phase, mice were conditioned for 3 days, and baseline data were collected. In the conditioning phase, mice were given once-daily alternating injections of either GBP or saline for 8 days and subsequently assessed in a post-conditioning test. Injections of 300 mg/kg of GBP significantly increased the time spent in the drug-paired chamber compared to the saline-paired chamber. However, lower doses of GBP (100 and 200 mg/kg) showed no effect. Pre-treatment with SKF-83566, a D1 receptor antagonist, attenuated GBP-induced CPP. Thus, for the first time, we show that GBP can induce CPP through a dopaminergic-dependent mechanism.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ibrahim Y, Hussain SM, Alnasser S, Almohandes H, Sarhandi I. Patterns and sociodemographic characteristics of substance abuse in Al Qassim, Saudi Arabia: a retrospective study at a psychiatric rehabilitation center. Ann. Saudi Med. 2018;38:319–325. doi: 10.5144/0256-4947.2018.319. - DOI - PMC - PubMed
-
- Evoy KE, Covvey JR, Peckham AM, Ochs L, Hultgren KE. Reports of gabapentin and pregabalin abuse, misuse, dependence, or overdose: An analysis of the Food And Drug Administration Adverse Events Reporting System (FAERS) Res. Soc. Adm. Pharm. 2019;15:953–958. doi: 10.1016/j.sapharm.2018.06.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

