Long-term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results
- PMID: 32591783
- PMCID: PMC8311580
- DOI: 10.1093/ndt/gfaa117
Long-term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results
Abstract
Background: Anti-CD20 B-cell depletion has not shown superior efficacy to standard immunosuppression in patients with systemic lupus erythematosus (SLE). Besides trial design, potential explanations are incomplete B-cell depletion in relation to substantial surges in B-cell-activating factor (BAFF). To improve B-cell targeting strategies, we conducted the first study in SLE patients aimed at investigating immunological effects and feasibility of combining rituximab (RTX; anti-CD20) and belimumab (BLM; anti-BAFF).
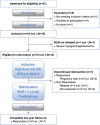
Methods: Reported is the long-term follow-up of a Phase 2 proof-of-concept study in 15 patients with SLE including 12 (80%) with lupus nephritis (LN).
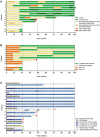
Results: In 10/15 (67%) patients, a clinical response was observed by achievement of lupus low disease activity state, of which 8 (53%) continued treatment (BLM + ≤7.5 mg prednisolone) for the complete 2 years of follow-up. Five patients (33%) were referred to as 'non-responders' due to persistent LN, major flare or repetitive minor flares. Out of 12 LN patients, 9 (75%) showed a renal response including 8 (67%) complete renal responders. All anti-dsDNA+ patients converted to negative, and both anti-C1q and extractable nuclear antigen autoantibodies showed significant reductions. CD19+ B cells showed a median decrease from baseline of 97% at 24 weeks, with a persistent reduction of 84% up to 104 weeks. When comparing responders with non-responders, CD20+ B cells were depleted significantly less in non-responders and double-negative (DN) B cells repopulated significantly earlier.
Conclusions: Combined B-cell targeted therapy with RTX and BLM prevented full B-cell repopulation including DN B cells, with concomitant specific reduction of SLE-relevant autoantibodies. The observed immunological and clinical benefits in a therapy-refractory SLE population prompt further studies on RTX + BLM.
Keywords: autoantibodies; autoimmune glomerulonephritis; immune complex-mediated membranoproliferative; lupus nephritis; rituximab/belimumab; systemic lupus erythematosus.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

