In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19
- PMID: 32592657
- PMCID: PMC7311917
- DOI: 10.1016/j.cmet.2020.06.015
In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19
Abstract
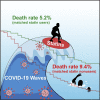
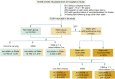
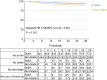
Statins are lipid-lowering therapeutics with favorable anti-inflammatory profiles and have been proposed as an adjunct therapy for COVID-19. However, statins may increase the risk of SARS-CoV-2 viral entry by inducing ACE2 expression. Here, we performed a retrospective study on 13,981 patients with COVID-19 in Hubei Province, China, among which 1,219 received statins. Based on a mixed-effect Cox model after propensity score-matching, we found that the risk for 28-day all-cause mortality was 5.2% and 9.4% in the matched statin and non-statin groups, respectively, with an adjusted hazard ratio of 0.58. The statin use-associated lower risk of mortality was also observed in the Cox time-varying model and marginal structural model analysis. These results give support for the completion of ongoing prospective studies and randomized controlled trials involving statin treatment for COVID-19, which are needed to further validate the utility of this class of drugs to combat the mortality of this pandemic.
Keywords: ACEi/ARB; COVID-19; SARS-COV-2; mortality; statin.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

Comment in
-
Impact of statins in patients with COVID-19.Rev Esp Cardiol (Engl Ed). 2021 Jul;74(7):637-640. doi: 10.1016/j.rec.2021.01.005. Epub 2021 Jan 21. Rev Esp Cardiol (Engl Ed). 2021. PMID: 33593686 Free PMC article. No abstract available.
References
-
- Calfee C.S., Delucchi K.L., Sinha P., Matthay M.A., Hackett J., Shankar-Hari M., McDowell C., Laffey J.G., O’Kane C.M., McAuley D.F., Irish Critical Care Trials Group Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018;6:691–698. - PMC - PubMed
-
- Craig T.R., Duffy M.J., Shyamsundar M., McDowell C., O’Kane C.M., Elborn J.S., McAuley D.F. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am. J. Respir. Crit. Care Med. 2011;183:620–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

