Pronounced Therapeutic Benefit of a Single Bidirectional AAV Vector Administered Systemically in Sandhoff Mice
- PMID: 32592687
- PMCID: PMC7544971
- DOI: 10.1016/j.ymthe.2020.06.021
Pronounced Therapeutic Benefit of a Single Bidirectional AAV Vector Administered Systemically in Sandhoff Mice
Abstract
The GM2 gangliosidoses, Tay-Sachs disease (TSD) and Sandhoff disease (SD), are fatal lysosomal storage disorders caused by mutations in the HEXA and HEXB genes, respectively. These mutations cause dysfunction of the lysosomal enzyme β-N-acetylhexosaminidase A (HexA) and accumulation of GM2 ganglioside (GM2) with ensuing neurodegeneration, and death by 5 years of age. Until recently, the most successful therapy was achieved by intracranial co-delivery of monocistronic adeno-associated viral (AAV) vectors encoding Hex alpha and beta-subunits in animal models of SD. The blood-brain barrier crossing properties of AAV9 enables systemic gene therapy; however, the requirement of co-delivery of two monocistronic AAV vectors to overexpress the heterodimeric HexA protein has prevented the use of this approach. To address this need, we developed multiple AAV constructs encoding simultaneously HEXA and HEXB using AAV9 and AAV-PHP.B and tested their therapeutic efficacy in 4- to 6-week-old SD mice after systemic administration. Survival and biochemical outcomes revealed superiority of the AAV vector design using a bidirectional CBA promoter with equivalent dose-dependent outcomes for both capsids. AAV-treated mice performed normally in tests of motor function, CNS GM2 ganglioside levels were significantly reduced, and survival increased by >4-fold with some animals surviving past 2 years of age.
Keywords: AAV9; GM2 gangliosidosis; Sandhoff disease; Tay-Sachs disease; gene therapy; intravenous delivery.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures
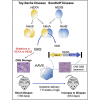


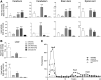

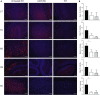

Comment in
-
Dual Purpose Vectors for Rare Neurological Diseases.Mol Ther. 2020 Oct 7;28(10):2104-2105. doi: 10.1016/j.ymthe.2020.09.017. Epub 2020 Sep 18. Mol Ther. 2020. PMID: 32949496 Free PMC article. No abstract available.
References
-
- Jeyakumar M., Thomas R., Elliot-Smith E., Smith D.A., van der Spoel A.C., d’Azzo A., Perry V.H., Butters T.D., Dwek R.A., Platt F.M. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain. 2003;126:974–987. - PubMed
-
- von Specht B.U., Geiger B., Arnon R., Passwell J., Keren G., Goldman B., Padeh B. Enzyme replacement in Tay-Sachs disease. Neurology. 1979;29:848–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

