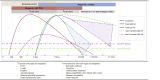
Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results
- PMID: 32593741
- PMCID: PMC7315992
- DOI: 10.1016/j.cmi.2020.06.019
Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results
Abstract
Background: To face the current COVID-19 pandemic, diagnostic tools are essential. It is recommended to use real-time RT-PCR for RNA viruses in order (a) to perform a rapid and accurate diagnostic, (b) to guide patient care and management and (c) to guide epidemiological strategies. Further studies are warranted to define the role of serological diagnosis and a possible correlation between serological response and prognosis.
Objectives: The aim was to guide clinical microbiologists in the use of these diagnostic tests and clinicians in the interpretation of their results.
Sources: A search of literature was performed through PubMed and Google Scholar using the keywords SARS-CoV-2, SARS-CoV-2 molecular diagnosis, SARS-CoV-2 immune response, SARS-CoV-2 serology/antibody testing, coronavirus diagnosis.
Content: The present review discusses performances, limitations and use of current and future diagnostic tests for SARS-CoV-2.
Implications: Real-time RT-PCR remains the reference method for diagnosis of SARS-CoV-2 infection. On the other hand, notwithstanding its varying sensitivity according to the time of infection, serology represents a valid asset (a) to try to solve possible discrepancies between a highly suggestive clinical and radiological presentation and negative RT-PCR, (b) to solve discrepancies between different PCR assays and (c) for epidemiological purposes.
Keywords: COVID-19; Coronavirus; Molecular diagnostic testing; Reverse transcriptase polymerase chain reaction; SARS-COV-2; Serology.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- WHO . 2020. WHO characterizes COVID-19 as a pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-a... Available from: (cited 25 March 2020)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous